One of the oldest and most important plant-based secondary metabolites utilized in cancer treatment is vinca alkaloids, which are extracted from the Catharanthus roseus plant. These chemicals, which include vindesine, vinblastine, vincristine, and vinorelbine, have a unique method of action in which they interfere with microtubule dynamics, causing malignant cells to apoptosis and cell cycle arrest. Vinca alkaloids' flexibility is proven by their usage in the therapy of haematological along with non-malignant conditions, and their role in cancer. Their biosynthesis, mode of action, pharmacological uses, and therapeutic difficulties are examined in this review. In order to reduce toxicity and improve efficacy, it also discusses new developments in synthetic modifications and drug delivery techniques. This article emphasizes the continued importance of vinca alkaloids in creating tailored cancer therapeutics by highlighting the meeting point of contemporary pharmacology and traditional medicine.
Cancer, Alkaloids, Biosynthesis, Vinca, Catharanthus Roseus, Extraction
Cancer ranks as one of the top reasons of death across globe. Cancer, defined as the uncontrolled, rapid generation of abnormal cells caused by various alterations in the regulation of genes is one among the primary causes of death due to illness globally. It killed over 10 million people worldwide in 2020, with breast cancer, colon cancer, lung cancer, and rectal cancer, skin cancer, prostate cancer, and stomach cancer accounting for a high proportion of new cases (https://www.who.int/news-room/fact sheets/detail/cancer). Chemotherapy is harmful to healthy cells, and chemically generated medications, radiotherapy, and chemotherapy are now separate components of cancer treatment (Zlatian OM.et.al., 2015, Buga AM.et.al., 2019, Zijlstra A.et.al., 2019). Ninety percent of high-income nations typically offer comprehensive cancer care, whereas only fifteen percent of low-income countries do so. This results in a significant financial burden on cancer therapy (WHO, 2020). Cancer was projected to have cost the US economy $1.16 trillion in 2010 alone (Wild C. et al., 2020). However, if identified early and treated appropriately, many tumors can be cured. Eliminating associated risks while employing evidence-based preventative strategies may avoid 30 to 50 % of cancers (https://www.who.int/news-room/fact-sheets/detail/cancer). Plant secondary metabolism products are well-classified and regarded as bioactive chemicals for preventing cancer at both the primary and secondary levels. As a result, numerous pieces of data demonstrate that consuming more secondary metabolites can reduce the risk of developing cancer (Kato Y. et al., 2018). These chemicals inhibit the formation and death of microtubule assemblies in cells, as well as the regulation of metabolic and signaling pathways that drive angiogenesis. Secondary metabolites are classified into the following main categories: lignans, steroids, phenolics, terpenes, glucosides, curcumins, saponins, alkaloids along with flavonoids (Sharifi-Rad J. et al., 2022). Because these plant-based bioactive molecules offer much-needed geno-protective actions, such as preserving healthy cells from DNA damage, secondary metabolites, either individually or in groups, can be employed to construct personalized cancer prevention strategies (Islam MT.et.al.,2021, Hossain R.et.al.,2022, Salehi B.et.al., 2019). Alkaloids are a family of phytochemicals that have showed potential as anticancer agents.
Alkaloids:
Alkaloids are a varied group of chemicals; around 3000 alkaloids from plants, fungi, and mammals have been identified. Among the best-known alkaloids are nicotine and morphine. According to Kurek et al. (2019), alkaloids are low molecular weight organic nitrogenous substances that are commonly classified chemically as tropanes, quinolines, terpenoids, isoquinolines, pyrrolidines, indoles, pyrrolizidines, and steroids. The alkaloids are typically crystalline, colorless, and non-volatile (https://www.britannica.com/science/atropine). They are also thought to be more stable and less harmful. Alkaloids have been scientifically demonstrated to limit the topoisomerase enzyme, reducing cell death and DNA replication. Alkaloids has therefore served as the foundation for the development of medications for a variety of diseases, comprising anti-inflammatory, antibacterial, along with anticancer properties (Dey P. et al., 2020).Alkaloids derived from plants have been beneficial in reducing oncogenesis. Alkaloids, a varied category of organic molecules found naturally, make up the bulk of the biggest group of phytochemicals (Kurek J. et al., 2019). One important characteristic that sets alkaloids apart and contributes to their alkaline qualities and therapeutic effects is their nitrogen atoms. The majority of alkaloids are synthesized using amino acids such lysine, tyrosine, phenylalanine, ornithine, along with tryptophan. Numerous alkaloids are produced as a result of the transformation of these precursors into a range of core intermediates. Most alkaloids have heterocyclic tertiary nitrogen in their structure, though they can take many various chemical forms. Alkaloids, which are largely generated from plants, number about 20,000. Alkaloids have also been found to be present in microorganisms and also in marine organisms such as puffer fish, dinoflagellates, as well as algae, as well as terrestrial animals like toads and insects. Alkaloids are found in plant species that have more than 0.001% alkaloids. Plant families such as Papaveraceae, Apocynaceae, Solanaceae, Amaryllidaceae, Asteraceae, Rutaceae, Fabaceae, along with Rubiaceae all have the potential for pharmacological usage.Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).
Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines,
such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic
acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014)Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic
acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014)Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).
Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001). Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole
structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and
proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines,such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014)Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes, inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanismsof action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014) Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines,such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014)Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012). Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acidsynthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes, inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrolestructures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic
acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic
acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes, inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001). Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic
acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic
acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012).Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001).Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by
perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic
acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014). Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes,
inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012). Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001). Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acid
synthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014). Alkaloids affect different metabolic systems in animals, and the toxic mechanism of action of alkaloids may vary considerably. Toxicity may arise by enzymatic alterations affecting physiological processes, inhibition of DNA synthesis and repair mechanisms by intercalating with nucleic acids, or affecting the
nervous system. Several alkaloids may affect multiple functions (Mithöfer and Boland 2012). Taxines are calcium channel antagonists, increasing cytoplasmic calcium (Wilson et al. 2001). Pyrrolizidine alkaloid toxic effects are mainly due to their biotransformation into strong reactive pyrrole structures by oxidases from the mammalian liver. The reactive pyrroles act by alkylating nucleic acids and proteins (Cushnie et al. 2014). Alkaloid mechanisms of action as antibacterial agents differ among alkaloid classes. Synthetic quinolone alkaloids may have respiratory inhibition effects; isoquinolines, such as berberine, sanguinarine, protoberberine, and benzophenanthridine, inhibit cell division by perturbing the Z-ring; the phenanthridine isoquinoline alkaloid ungeremine acts by inhibiting nucleic
acid synthesis; pergularinine and tylophorinidine, which are indolizidine alkaloids, inhibit nucleic acidsynthesis as well, by targeting dihydrofolate reductase (Cushnie et al. 2014).
CLASSIFICATION:
Pyrolidine alkaloids, imidazole, tropane, indole, piperidine, purine, pyrrolizidine, quinolozidine, as well as isoquinoline are among the chemicals classified according to their heterocyclic ring system and biosynthetic precursors (Dey P. et al., 2020). Numerous researchers have classed alkaloids in different ways. One of the most often used categorization techniques divides an entire class of chemicals into three categories.
1.True Alkaloids:
True alkaloids, which include atropine and nicotine, are composed of an amino acid and a heterocyclic ring containing nitrogen.
2. Proto Alkaloids:
Proto-alkaloids include adrenaline, ephedrine, and other compounds with a nitrogen atom derived from an amino acid that is not a component of the heterocyclic ring.
3. Pseudo Alkaloids:
Caffeine, theobromine, and other substances that do not come from amino acids are known as pseudo-alkaloids (Dey P. and others, 2020).
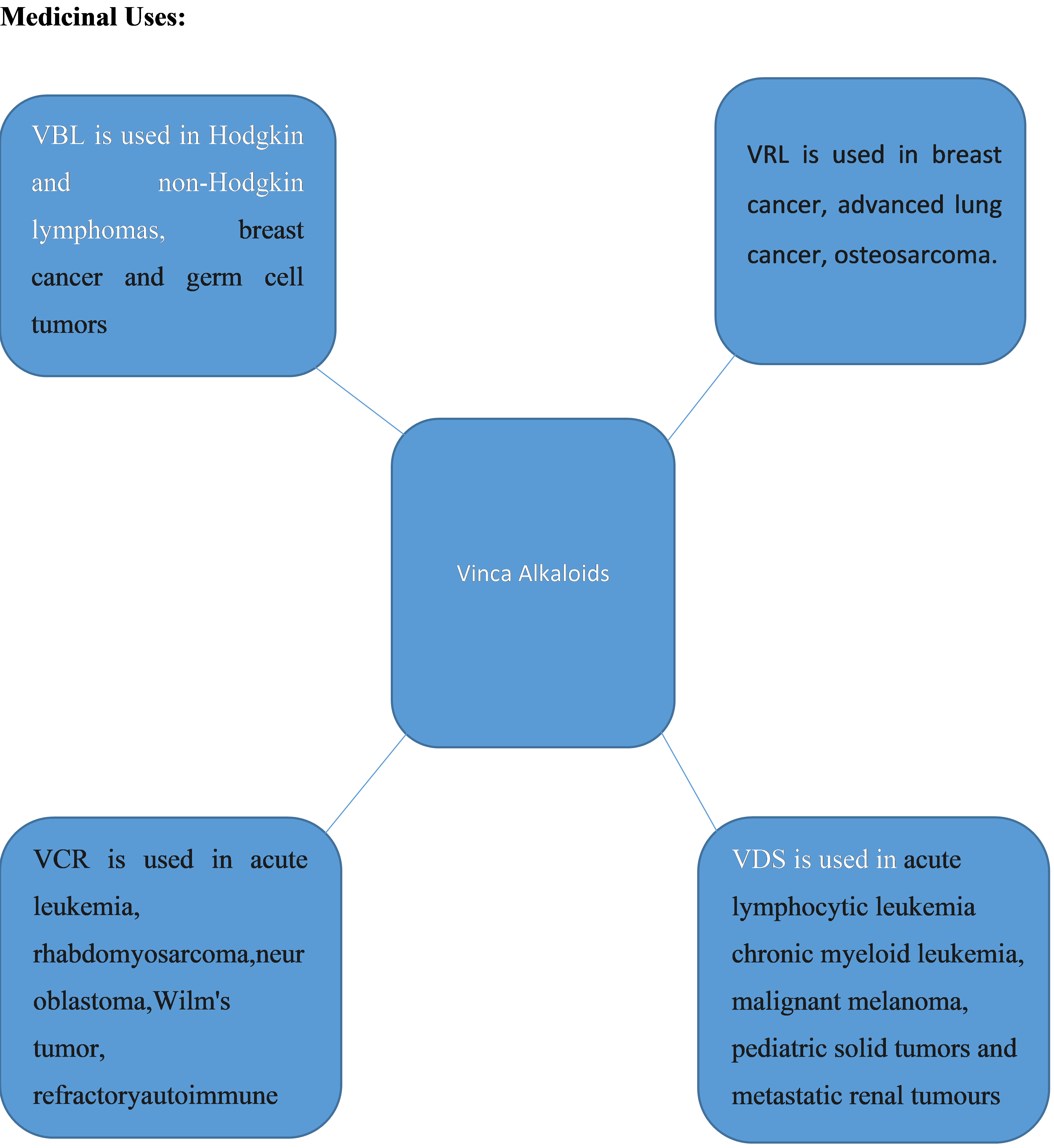
Vinca Alkaloids:
Vinca alkaloids are a type of organic molecule known as alkaloids. They are commonly produced by plants and are made up of hydrogen, oxygen, nitrogen and carbon.

Fig No Vinblastine
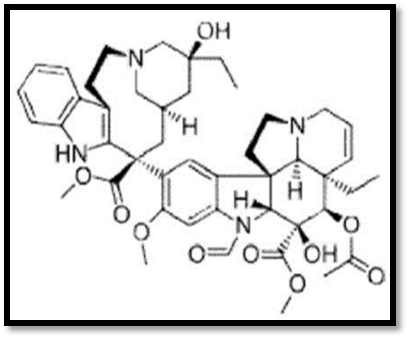
Fig No: Vincristine
However, the name symbolizes alkali, such as those substances that don't have alkaline qualities. Despite their toxic properties, many alkaloids also have physiological effects that make them effective medications (Sahelian R. et al., 2011).Vinca alkaloids are the very old type of plant utilizing for treating cancer (Brogan C., 2010).
Source:
Vinca alkaloids are obtained from the periwinkle plant in Madagascar. The pink periwinkle plant Catharanthus roseus G. Don creates naturally occurring or partially synthetic nitrogenous bases(Kufe DW.et.al., 2003). Canadian scientists Robert Noble with Charles Beer made the initial discovery of vinca alkaloids in the 1950s.
Mechanism of Action:
Vince alkaloids' major means of cytotoxicity involve their relationships between tubulin and the disruption of microtubule function, namely of the microtubules that constitute the spindle of mitosis machinery, which culminates in metaphase arrest. They can, however, engage in a wide range of additional metabolic processes that could or might not be connected to their impact on microtubules. Many of the consequences that do not involve microtubule breakdown become apparent only after cells have undergone treated with clinically modest concentrations of vinca alkaloids. However, because microtubules play a role in a variety of non-mitotic processes, vinca alkaloids and other antimicrotubule medications affect both malignant and non-malignant cells throughout the non-mitotic cycle. Vinca alkaloids bind to tubulin binding sites that differ from those found in guanosine-5'-triphosphate, podophyllotoxin, colchicine, and taxanes. KH Downing et al. (2000). Binding happens quickly and can sometimes go the other way. The data currently available supports two vinca alkaloid binding sites per mole of tubulin dimer(Correia JJ. et al., 2001). Each microtubule end has around 16-17 high-affinity binding locations. Vinca alkaloids bind to these sites, interrupting microtubule assembly; nonetheless, one of the most significant effects of small amounts of drug could be a reduction in the rates of microtubule development and shorten at the structure's end, resulting in a "kinetic cap" and functional suppression ( Ann Jordan M. et al., 1992). At lower pharmacological dosages than those that reduce microtubule mass, vinca alkaloids disrupt microtubule dynamics, specifically at the mitotic spindle's extremities, causing metaphase arrest (Toso RJ. et al., 1993). In vitro, vinca alkaloids and other microtubule-disrupting drugs can inhibit malignant angiogenesis. At dosages ranging from 0.1 to 1.0 pmol/L, VBL reduced endothelial proliferation, chemotaxis, and fibronectin spreading—all of which are essential processes in angiogenesis. At these low concentrations, normal fibroblasts and lymphoid malignancies were unaffected. Low dosages of VBL combined with vascular endothelial growth factor antibodies significantly increased the anticancer response, particularly in cancers resistant to the drug's direct cytotoxic effects (Klement G. et al., 2000). Vinca alkaloids inhibit cell division via attaching to microtubules, which may result in apoptosis and a mitotic halt. VCR and similar compounds destabilize microtubules by attaching to tubulin and preventing polymerization (Wang LG.et.al., 1999).
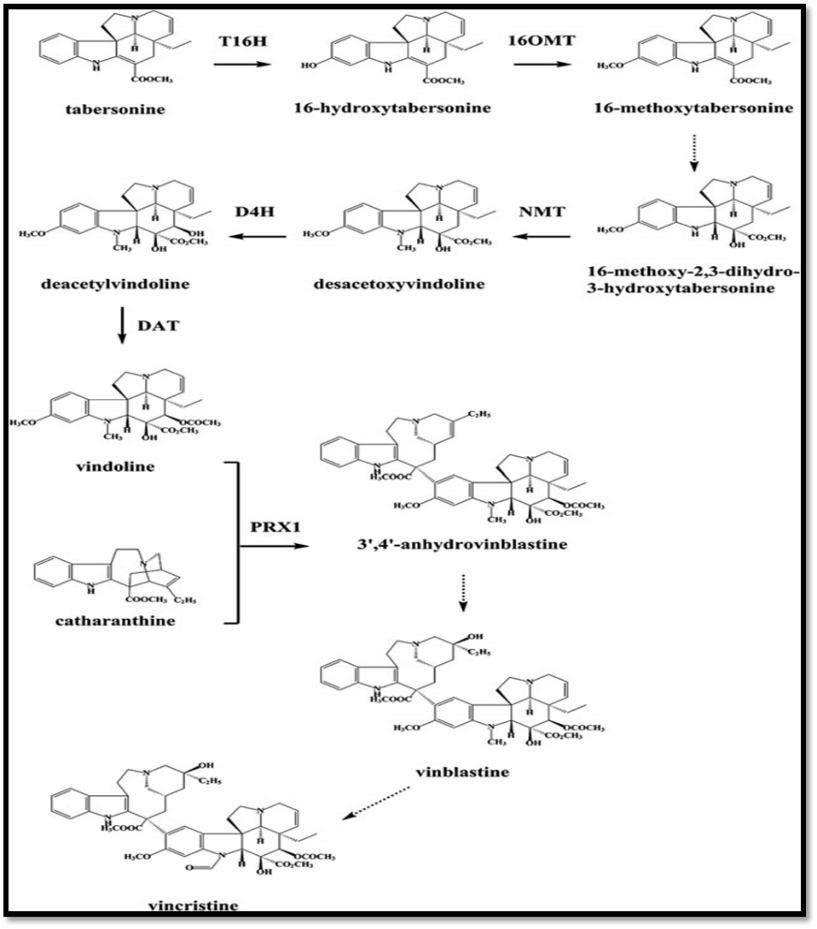
Biosynthesis of Vinca Alkaloids:
The biosynthesis of vincristine and vinblastine, two significant anticancer alkaloids made from tabersonine and catharanthine, is depicted in this image:
1. Using the T16H enzyme, tabersonine converts to 16-hydroxytabersonine, and using the 16OMT enzyme, it converts to 16-methoxytabersonine.
2. NMT enzyme ? desacetoxyvindoline ? deacetylvindoline ? vindoline (via DAT enzyme) ? 16-methoxytabersonine ? 16-methoxy-2,3-dihydro-3-hydroxytabersonine (by several stages).
3. 3',4'-anhydrovinblastine is created when vindoline and catharanthine interact (via the PRX1 enzyme).
4. After being transformed into vinblastine, 3',4'-anhydrovinblastine undergoes further modification to yield vincristine.Certain enzymes (e.g., T16H, 16OMT, PRX1) catalyze each step.


 Satyam Ambardekar* 1
Satyam Ambardekar* 1




 10.5281/zenodo.14695718
10.5281/zenodo.14695718