Abstract
Oxidative stress results from an imbalance between reactive oxygen species(ROS)production and the body’s antioxidant defenses, leading to damage of proteins, lipids, cell membranes, and DNA. Studies has demonstrated that oxidative stress is a crucial factor in various pathological events, particularly in diabetic complications, neurological disorders (CNS particularly) and cancer. Glutamate, the primary excitatory neurotransmitter in the central nervous system (CNS), plays a critical role in neural communication, plasticity, and energy metabolism. Cortisol is a glucocorticoid hormone produced and released by the adrenal glands which has profound effect on stress and metabolic reactions. This systematic review examines the interplay between cortisol and glutamate synthesis during oxidative stress, focusing on molecular pathways, regulatory feedback, and implications for neurodegenerative diseases and stress-related disorders. Findings underscore the dual role of cortisol in maintaining metabolic balance and contributing to excitotoxicity under chronic stress, offering insight into therapeutic targets for oxidative damage mitigation.
Keywords
CNS (Central Nervous System), ROS (Reactive Oxygen Species), neuroplasticity, neurodegenerative, TCA (Tricarboxylic Acid Cycle).
Introduction
Oxidative stress is a biological process triggered by an imbalance between the generation of free radicals, especially reactive oxygen species (ROS), and the body’s ability to neutralize these harmful compounds with antioxidants (Ana Salomé Correia and Nuno Vale, 2024). Reactive oxygen species (ROS) are partially reduced, reactive forms of oxygen; they include superoxide, hydroperoxyl radical, hydroxyl radical, peroxynitrite, and hypochlorous. ROS are increased during oxidative stress and inflammation and cause damage to all biomolecules. The balance between the free radicals of ROS and the body antioxidant is very crucial for cellular processes in the body. While ROS function has essential signaling molecules at normal levels, their overproduction during oxidative stress can cause significant cellular damage. This damage results from harmful interactions with proteins, lipids, and DNA, contributing to the development of numerous oxidative stress-related conditions such as cancer, diabetes and obesity (metabolic disorder), Alzheimer’s disease and Parkinson’s disease (neurodegenerative disease) and skin related disorders such as acne, eczema and vitiligo etc. Smoking and ultraviolet (UV) radiation further exacerbate ROS production, leading to cellular damage. Cortisol and glutamate are crucial for understanding the brains adaptive and maladaptive responses to oxidative stress. Glutamate, synthesized through multiple pathways, is essential for synaptic plasticity and energy metabolism. Furthermore, glutamine, a derivative of glutamate is known to have a role in the maintenance of the skin structure, hydration and repair which makes it a valuable component of skin care. Cortisol on the other hand, the most abundant glucocorticoid in the human body produced in the adrenal cortex known as the stress hormone. Cortisol in the body responds to elevated stress levels and other physiological processes such as carbohydrate metabolism, blood pressure regulation etc. in the body. Cortisol also, has a role in the transcription of neuroplasticity. Furthermore, “as a lipophilic hormone cortisol is capable of freely crossing cell membranes, binding to glucocorticoid receptors (GR) in the cytoplasm of neuronal cells. This binding forms an hormone-receptor complex that translocates into the nucleus, acting as a transcription factor by binding to glucocorticoid-responsive elements” (GREs) in DNA (João Carlos Orquiza,2024). This complex modulates the expression of several genes that influence neuronal metabolic pathways, directly impacting synaptic plasticity and neuronal resilience (Sapolsky, 2000). Neuronal plasticity depends on the continuous production of ATP in which “cortisol regulates the balance between ATP consumption and production in neurons by modulating genes that affect mitochondrial functionality and glucose availability”(João Carlos Orquiza,2024). This review will systematically explores the role of cortisol in glutamate synthesis under oxidative stress, focusing on implications for neuronal health and systematic damage.
METHODS
This systematic review utilized databases such as Osmosis from Elsevier, the National Library of medicine, and other academic sources to identify peer-reviewed articles and clinical studies related to cortisol, glutamate, and oxidative stress. Key search terms included “Cortisol, “Glutamate synthesis,”and Oxidative stress.”
RESULTS
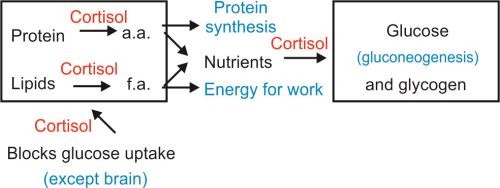
- Cortisol in cellular metabolism: Cortisol belongs to the glucocorticoid class of hormones produced by a pair of adrenal glands that are located above each kidney. Cortisol lipophilic nature allows it to diffuse across cell membranes, binding to glucocorticoid receptors(GRs) in the cytoplasm. Once inside the cell, cortisol binds with its protein receptor in the cytoplasm, and the hormone-receptor complex then interacts with specific regulatory DNA sequences, called glucocorticoid response elements, to induce or repress gene transcription. Cortisol affects protein, fat, and carbohydrate metabolism in a coordinated fashion to increase glucose synthesis as follows; Cortisol increases protein catabolism in muscle and decreases new protein synthesis, thereby providing additional amino acids to the liver for gluconeogenesis. Cortisol increases lipolysis, which provides additional glycerol to the liver for gluconeogenesis ensuring glucose availability during stress. Furthermore, dysregulation of cortisol contributes to metabolic disorders and chronic inflammation. We have provided a schematic diagram to show the role of cortisol in cellular metabolism.
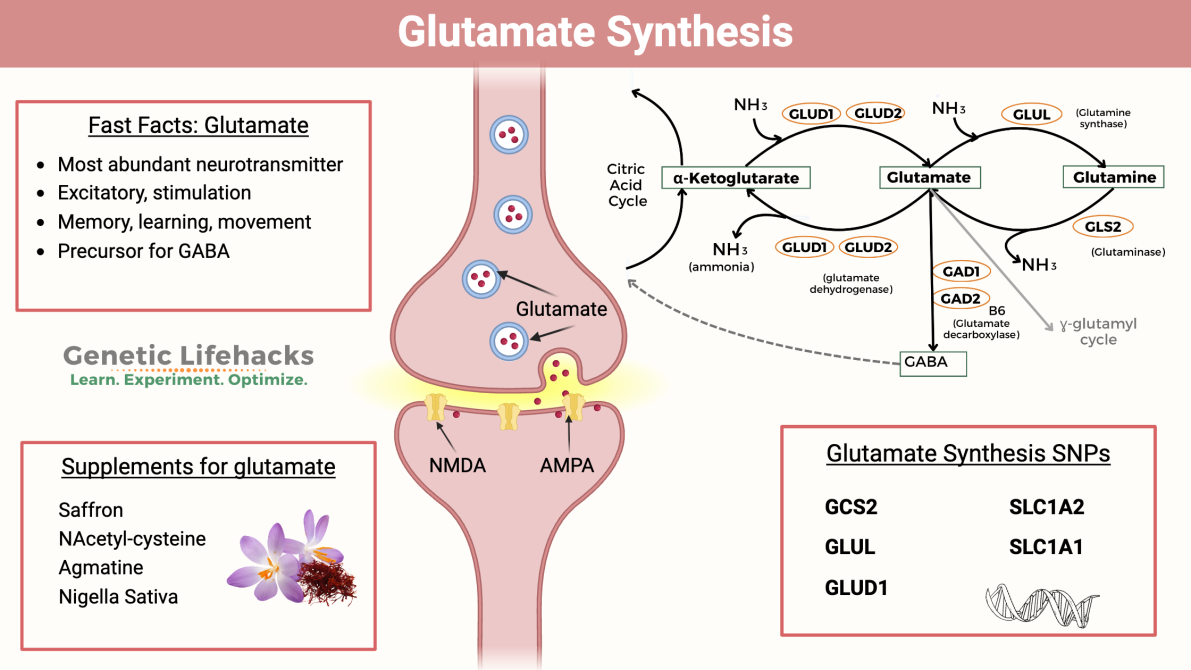
- Glutamate synthesis under oxidative stress: Glutamate is synthesized from alpha ketoglutarate in the TCA cycle or from glutamine through the action of glutaminase. Glutamate is a non-essential amino acid that does not cross the brain-blood barrier and must be synthesized in neurons from local precursors. Glutamine is released by glial cells and once within presynaptic terminals, is metabolized to glutamate by the mitochondrial enzyme glutaminase. Glutamate plays key roles linking carbohydrate and amino acid metabolism via the tricarboxylic acid (TCA) cycle, as well as in nitrogen trafficking and ammonia homeostasis in the brain. During oxidative stress, disruptions in reactive oxygen species(ROS) clearance impair glutamate homeostasis, leading to excitotoxicity and neuronal damage. Additionally, amino acids are known for their ability to promote the production of collagen, fibrinogen and elastin. Collagen prevents skin sagging by ensuring the skin stays strong and maintains elasticity. Collagen is also essential for the skin’s natural renewal process, therefore eating foods rich in proteins or utilising products containing collagen amino acids is recommended. Amino acids such as glutamine, a derivative of glutamate produces antioxidants and protects the skin from impurities. To elaborate the synthesis of glutamate we have provided a schematic diagram for further explanation.
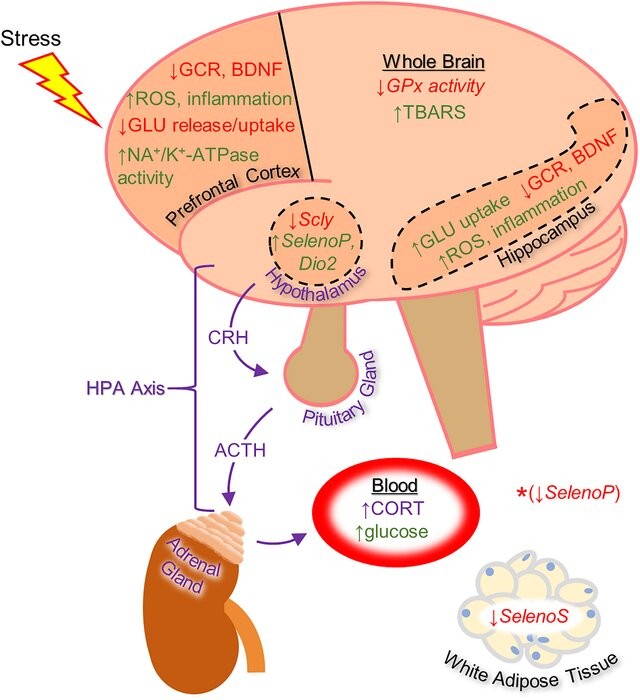
3. Cortisol role in modulating glutamate level during oxidative stress: Cortisol secretion during acute stress affect the basal release of glutamate in several limbic and cortical areas, including the hippocampus, amygdala and prefrontal cortex. Converging lines of evidence from animal studies suggest that acute exposure to stress or administration of glucocorticoids rapidly increases glutamate release in these brain areas. In different studies using patch-clamp recordings, application of 100 nM corticosterone, the major glucocorticoid (in rodents), to hippocampal slices rapidly enhanced the frequency of miniature excitatory postsynaptic potentials in CA1 pyramidal neurons and reduced paired-pulse facilitation (PPF) — a form of synaptic facilitation that reflects presynaptic release — suggesting that corticosterone increases glutamate release probability in this areas of the brain. Acute stress enhances glutamate release, mediated through non-genomic pathways involving mineralocorticoid receptors. Chronic elevation of cortisol, leads to glutamate accumulation, excitotoxicity, and oxidative damage, underscoring its dual role in maintaining homeostasis and contributing to pathology. The diagram below is provided to show the interplay between cortisol and glutamate during acute stress.
DISCUSSION
- Neurodegenerative effect: Excess glutamate in the CNS can lead to excitotoxity, which is influenced by the dysregulation of cortisol, which can lead to neuronal damage and death. While cortisol, under normal conditions, supports glutamate clearance via astrocytic uptake and glutamine recycling, chronic cortisol elevation disrupts this balance, contributing to oxidative stress and neurodegeneration.
- Chronic psychological stress: Chronic psychological stress appears to accelerate biological aging, and oxidative damage which is an important potential mediator of this process (Kirstin Aschbacher et al,2013). “During periods of intense stress, people are sometimes said to have ‘‘aged before your eyes.’’ This popular idiom reflects the widespread cultural belief that chronic psychological stress can accelerate the aging process. Moreover, it is consistent with accumulating evidence that individuals exposed to chronic stress show signs of accelerated biological aging, such as systemic inflammation and shorter telomere length” (Epel et al., 2004; Damjanovic et al., 2007; Gouin et al., 2008; Humphreys et al., 2012). Cortisol, as a central stress hormone, amplifies oxidative stress when dysregulated, further impacting neuronal plasticity and resilience. Recent research has highlight the need for interventions targeting cortisol regulation to mitigate stress-induced neurodegeneration and systemic effects.
- Effect of oxidative stress on the skin: The skin is the largest organ that regulates excretion of metabolic waste products, temperature, and plays an important role in body protection against environmental, physical and chemical, as well as biological factors. Exposure of the skin to UV radiation or environmental factors including chemicals (e.g. xenobiotics) is the next efficient source of ROS (Joanna kruk and Ewa Duchnik, 2014). These include agents that may act as oxidants or catalysts of reactions producing reactive oxygen species (ROS), reactive nitrogen species (RNS), and other oxidants in skin cells. ROS leads to chronic inflammation, which, in turn, can cause collagen fragmentation and disorganization of collagen fibers and skin cell functions, and thus contribute to skin diseases including cancer (Joanna kruk and Ewa Duchnik, 2014). According to the research studies carried out by Liya Jiang, Zhen Guo et al in 2018 to investigate the protective effects of glutamine on a human melanocyte oxidative stress model, it was noted that glutamine enhances the antioxidant and anti-apoptotic capabilities of melanocytes and protects them against oxidative stress. For skin disease such as vitiligo, studies have shown that oxidative-stress induced apoptosis in melanocytes is closely related to the pathogenesis of vitiligo. Moreover, research suggests that oxidative stress participates in all stages of carcinogenesis, therefore, future studies should explore glutamine supplementation as a strategy for managing oxidative stress-related skin disorders.
- Therapeutic implications: Targeting the cortisol-glutamate axis represents a promising avenue for mitigating oxidative damage. Pharmacological modulation of cortisol levels, combined with interventions to regulate glutamate clearance and recycling, may help restore neuronal homeostasis and reduce excitotoxicity. Antioxidant therapies that enhances cellular resilience to ROS also hold potential in addressing both neurodegenerative disease and stress-related conditions.
CONCLUSION
Oxidative stress is a key contributor to numerous physiological and psychological disorders, with cortisol and glutamate playing pivotal roles in its pathology. This review highlights the complex relationship between these factors, emphasizing the need for targeted interventions to address dysregulation. Future research should focus on providing precise mechanisms and developing integrated therapeutic strategies to combat oxidative damage effectively.
REFERENCE
- Aschbacher, Kirstin & O'Donovan, Aoife & Wolkowitz, Owen & Dhabhar, Firdaus & Su, Yali. (2013). Good Stress, Bad Stress and Oxidative Stress: Insights from Anticipatory Cortisol Reactivity. Psychoneuroendocrinology. 38. 10.1016/j.psyneuen.2013.02.004.
- Correia, Ana & Vale, Nuno. (2024). Exploring Oxidative Stress in Disease and Its Connection with Adenosine. Oxygen. 4. 325-337. 10.3390/oxygen4030019.
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470.S
- De Kloet ER, Karst H, Joels M. Corticosteroid hormones in the central stress response: quick-and-slow. Front Neuroendocrinol. 2008;29:268–272. doi: 10.1016/j.yfrne.2007.10.002. S0091-3022(07)00057-X [pii] [DOI] [PubMed] [Google Scholar][Ref list
- Epel, E.S., Blackburn, E.H., Lin, J., Dhabhar, F.S., Adler, N.E., Morrow, J.D., Cawthon, R.M., 2004. Accelerated telomere shortening in response to life stress. PNAS 101, 17312—17315.
- Epel, E.S., Lin, J., Dhabhar, F.S., Wolkowitz, O.M., Puterman, E., Karan, L., Blackburn, E.H., 2010. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav. Immun. 24, 531—539.
- Jiang L, Guo Z, Kong Y, Liang J, Wang Y, Wang K. Protective effects of glutamine on human melanocyte oxidative stress model. Indian J Dermatol Venereol Leprol. 2018 May-Jun;84(3):269-274. doi: 10.4103/ijdvl.IJDVL_106_17. PMID: 29491190.
- Karst H, et al. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. 0507572102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar][Ref list]
- Kruk, Joanna & Duchnik, Ewa. (2014). Oxidative Stress and Skin Diseases: Possible Role of Physical Activity. Asian Pacific journal of cancer prevention : APJCP. 15. 561-8. 10.7314/APJCP.2014.15.2.561.
- Lowy MT, Wittenberg L, Yamamoto BK. Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. Journal of Neurochemistry. 1995; 65: 268–274. doi: 10.1046/j.14714159.1995.65010268.x. [DOI] [PubMed] [Google Scholar][Ref list]
- Orquiza, João. (2024). cortisol: central regulator in neuronal plasticity. 10.5281/zenodo.14047275.
- PhD, J.W. B. (2022). Medical Biochemistry - E-Book (6th ed.). Elsevier Limited (UK). https://clinicalkeymeded.elsevier.com/books/9780323834513
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011 Nov 30;13(1):22-37. doi: 10.1038/nrn3138. PMID: 22127301; PMCID: PMC3645314.
- Sapolsky RM. Glucocorticoids and Hippocampal Atrophy in Neuropsychiatric Disorders. Arch Gen Psychiatry. 2000;57(10):925–935. doi:10.1001/archpsyc.57.10.925
- Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol. 2014; 11:13- 30. doi: 10.1007/978-3-319-08894-5_2. PMID: 25236722; PMCID: PMC4667713


 Sewanu Stephen Godonu *
Sewanu Stephen Godonu *
 Norrian Francis-Lyons
Norrian Francis-Lyons



 10.5281/zenodo.14606017
10.5281/zenodo.14606017