Abstract
Nanoemulsions are considered as the most promising solution to improve the delivery of ophthalmic drugs. The design of ophthalmic nanoemulsions requires an extensive understanding of pharmaceutical as well as technological aspects related to the selection of excipients and formulation processes. This Review aims at providing the readers with a comprehensive summary of possible compositions of nanoemulsions, methods for their formulation (both laboratory and industrial), and differences between technological approaches, along with an extensive outline of the research methods enabling the confirmation of in vitro properties, pharmaceutical performance, and biological activity of the obtained product. The composition of the formulation has a major influence on the properties of the final product obtained with low-energy emulsification methods. Increasing interest in high-energy emulsification methods is a consequence of their scalability important from the industrial perspective. Considering the high-energy emulsification methods, both the composition and conditions of the process (e.g., device power level, pressure, temperature, homogenization time, or number of cycles) are important for the properties and stability of nanoemulsions. It is advisible to determine the effect of each parameter on the quality of the product to establish the optimal process parameters’ range which, in turn, results in a more reproducible and efficient production.
Keywords
nanoemulsion,glaucomatretment,ophthalmicnanoemulsion ocular drug delivery,emulsification
Introduction
Basic information of nanoemulsions
Nanoemulsions are a type of emulsion where two or more immiscible liquids are mixed together and stabilized withsurfactants or emulsifiers. The resulting droplets have adiameter of less than 1000 nanometers (nm). A nanoemulsion is a type of emulsion where two or more immiscible liquids are mixed together and stabilized with surfactants or emulsifiers1. The resulting droplets have a diameter of less than 1000 nanometers (nm).In simpler terms, a nanoemulsion is a mixture of two or more liquids that don't normally mix, like oil and water, blended together in a way that creates tiny droplets that are stable and don't separate
- A Some key characteristic of nanoemulsions.
- Small droplet size: Typically ranges from 10-1000 nm
- Stability: Resists separation and settling due to the presence of surfactants.
- Optical clarity: Appears transparent or translucent due to the small droplet size.
- Improved solubility: Can solubilize both hydrophilic and lipophilic compounds.
Oil-in-water (O/W): Oil droplets dispersed in a water phase.Water-in-oil (W/O): Water droplets dispersed in an oil phase.2Depending on constituents and relative distribution of the internal dispersed phase/phases and the more ubiquitous continuous phase, nanoemulsions are termed as biphasic (O/W or W/O) or multiple nanoemulsions (W/O/W). Phase volume ratio (?) measures comparative volumes of internal and external phase comprising a nanoemulsion and determines its droplet.number and overall stability. Normally, phase present in greater volume becomes the external phase. To predict type of nanoemulsion formed under
- Pharmaceuticals: Improved drug delivery and bioavailability.
- Cosmetics: Enhanced skin penetration and moisturization.
- Food: Increased stability and bioavailability of nutrients.
Ophthalmic: Targeted delivery of medications to the eye.
- Drug delivery: Nanoemulsions can improve the solubility and bioavailability of poorly soluble drugs.
- Cancer treatment: Nanoemulsions can be used to deliver chemotherapeutic agents directly to cancer cells.
- Vaccine delivery: Nanoemulsions can be used to deliver vaccines and improve immune response.
- Topical formulations: Nanoemulsions can be used to improve the delivery of topical medications for skin conditions
- Eye drops: Nanoemulsions can be used to deliver medications for eye conditions, such as glaucoma and dry eye syndrome.
- Contact lens care: Nanoemulsions can be used to deliver cleaning and disinfecting agents for contact lenses.
- Advantages of nanoemulsions
- Physical Advantages
- Small droplet size: Increases surface area, enhancing absorption and bioavailability.
- Stability: Resists separation and settling, ensuring consistent delivery.
- Optical clarity: Appears transparent or translucent, making them suitable for topical applications.
- Improved solubility: Can solubilize both hydrophilic and lipophilic compounds.
- Increased stability: Resists separation and settling, ensuring consistent delivery.
- Improved bioavailability: Increases absorption and utilization of nutrients.
- Enhanced solubility: Solubilizes both hydrophilic and lipophilic compounds.
- Increased shelf life: Reduces oxidation and spoilage.
- Pharmaceutical Advantages
- Enhanced bioavailability: Increases absorption and utilization of active ingredients.
- Targeted delivery: Can be designed to target specific tissues or cells.
- Improved solubility: Enhances solubility of poorly soluble drugs.
- Reduced toxicity: Allows for lower doses, reducing side effects.
- Increased patient compliance: More convenient and comfortable to use.
- Reduced waste: Can be designed to reduce waste and improve sustainability.
- Improved efficacy: Increases absorption and utilization of active ingredients, reducing the need for repeat applications.
- Reduced environmental impact: Can be designed to reduce environmental impact by using natural ingredients and reducing packaging.
- Disadvantages of nanoemulsions:
- Physical Disadvantages
- Instability: Nanoemulsions can be unstable and separate over time.
- Ostwald ripening: Larger droplets can grow at the expense of smaller ones, leading to instability.
- Flocculation: Droplets can aggregate and settle, affecting stability and bioavailability.
- Creaming: Droplets can rise to the surface and form a cream layer, affecting stability.
- Pharmaceutical Disadvantages
- Limited scalability: Nanoemulsion production can be challenging to scale up.
- High energy requirements: Producing nanoemulsions can require high energy inputs.
- Surfactant toxicity: Some surfactants used in nanoemulsions can be toxic.
- Limited understanding of biological fate: The behavior of nanoemulsions in the body is not yet fully understood.
- Skin irritation: Some surfactants used in nanoemulsions can cause skin irritation.
- Limited stability: Nanoemulsions can be unstable in cosmetic formulations.
- Difficulty in formulation: Formulating nanoemulsions for cosmetics can be challenging.
- High cost: Producing nanoemulsions for cosmetics can be expensive.
- Limited understanding of nutritional impact: The impact of nanoemulsions on nutrition is not yet fully understood.
- Potential toxicity: Some nanoemulsion components can be toxic.
- Regulatory challenges: There are regulatory challenges associated with using nanoemulsions in food.
- Scalability issues: Producing nanoemulsions for food can be challenging to scale up.
- Limited understanding of ocular fate: The behavior of nanoemulsions in the eye is not yet fully understood.
- Potential toxicity: Some nanoemulsion components can be toxic to the eye.
- Difficulty in formulation: Formulating nanoemulsions for ophthalmic use can be challenging.
- High cost: Producing nanoemulsions for ophthalmic use can be expensive.
- Environmental Disadvantages
- Potential environmental impact: The impact of nanoemulsions on the environment is not yet fully understood.
- Difficulty in disposal: Disposing of nanoemulsions can be challenging.
- Limited biodegradability: Some nanoemulsion components may not be biodegradable.
- Potential harm to aquatic life: Nanoemulsions can potentially harm aquatic life.
- Industrial-Scale Production of Ophthalmic Nanoemulsions A well-designed, small-scale ophthalmic nanoemulsion manufacturing process may prove to be unreliable or nonreproducible on a larger scale if not effectively controlled during the up-scale of production. The scaling-up of the emulsification process and the examples of large-scale nanoemulsion production are still limited3,4. In most cases the loss of product stability after upscaling is due to the inability of industrial manufacturers to apply the same shear forces that were used in the preformulation phase. Therefore, the selection of the formulation technology and robust equipment for large-scale production is fundamental. Factors that may change during scaling-up include, but are not limited to, structural changes of API and auxiliary substances, changes in particle size of the dispersed phase, variations in drug content along the quality, and quantity of impurities5. Furthermore, the changes in manufacturing processes can lead to reduced stability or even degradation of the product. On the laboratory scale, once the nanoemulsioncomposition and shear forces are optimized, the average size of the droplets of the dispersed phase can be reduced to the nanometer range. The same level of stability during industrial production can only be achieved if the average droplets’ size of the oil phase remain unchanged.While obtaining a laboratory-optimized defined composition of nanoemulsion formulation on an industrial scale is usually uncomplicated, maintaining the required intensity of shear forces is much more challenging. High-pressure homogenization, microfluidization, and high-amplitude ultrasonic treatment are currently the leading methods used to produce the highest-quality nanoemulsions. Despite their disadvantages, for example, the need to prepare a droplet dispersion (pre-emulsion) with a size of 1–10 ?m using a rotor-stator colloid mill, HPH and microfluidization are currently the preferred technologies for industrial production of pharmaceutical nanoemulsions6. Both processes are energy-intensive, require high-maintenance (both cleaning and wear) expensive equipment, and a significant process redesign to enable aseptic production. Microfluidization provides some advantage over HPH because of the construction of the equipment that enables easier scale up by multiplication of microfluidization chambers. This, in turn, provides nanoemulsions with similar properties (globule size, PDI) as compared to formulations obtained at a laboratory scale. Furthermore, the equipment manufacturing companies provide solutions that enable scaling the nanoemulsion formulation process from laboratory through pilot to production scale. The available industrial microfluidization equipment also enables sterile production and packing in a single process.The ultrasonic nanoemulsification is also possible to scale-up. In the process where the driving force is acoustic cavitation generated by an ultrasonic homogenizer, the higher the ultrasound amplitude, the higher the production speed, and the better quality of the final product7,8. However, scaling-up the process requires enlarging the cavitation zone without losing its intensity. Industrial ultrasonic processors contain much larger ultrasonic sonotrodes than laboratory devices (larger ultrasonic horns) and are capable of generating
larger cavitation zones; therefore, they process much more material per unit of time as compared with laboratory sonicators. Furthermore, scaling-up the process requires the samecavitation intensity in the production environment as originally used in the laboratory. It means that to obtain reproducible results after scale-up, the sonotrode amplitudes in laboratory and industrial processors must be kept at the same level. Conventional industrial ultrasonic devices cannot provide high enough amplitudes for efficient nanoemulsification that compromise the product quality. Newly developed industrial ultrasonic devices that utylise so-called Barbell Horn Ultrasonic Technology (BHUT) provide the same high ultrasonic amplitudes and cavitation intensity as used during the laboratory phase of product development, enabling the achievement of reproducible results on an industrial scale.
- In vitro drug release from nanoemulsions
The increased ability to solubilize the sparingly soluble active substance in nanoemulsions results in longer release of an API from these systems, as compared with conventional drug forms (e.g., eye drops), enabling the achievement of the therapeutic effect using a lower dose of the drug and to decrease the number of systemic side effects9.The in vitro release study of the API from nanoemulsions allows determination of the release kinetics of the drug from a given formulation, which may provide preclinical data on the biodistribution and bioavailability of the drug into the eye. Sustained release formulations may provide the drug penetration into the deeper layers of the eye structure after application. Biorelevant methods of testing the release of ophthalmic products in vitro are still under development. Since there are no accepted compendial standards for this area we provide the overview of different noncompendial methods used for evaluation of drug release from ophthalmic nanoemulsions in this section10. In vitro drug release from these systems is currently being assessed using a variety of membrane diffusion techniques including simple dialysis methods, dialysis methods using a modified type I or II apparatus, and Franz diffusion cells. The aforementioned USP type II apparatus is preferred for testing the release of substances from ophthalmic nanoemulsions. In this method, the formulation (0.5 mL) is placed in a dialysis bag and installed in the beaker containing an acceptor medium. The release test is usually performed at 34 ± 0.5 °C or 37 ± 0.5 °C in 900 mL of phosphate buffer at pH 7.4, often with addition of 1% sodium lauryl sulfate (SLS) or a buffered saline solution (PBS) at pH 7.4 with rotational speed of blades set to 50 rpm. The test is carried out in 3 replications for 6 h11. The medium samples are withdrawn at specified intervals and the loss of the collected medium is replenished with a fresh buffer in order to maintain a constant fluid volume. The concentration of the active substance is determined using high-performance liquid chromatography (HPLC) or UV–vis spectroscopy.
During the release of the API from nanoemulsion, the drug diffuses from the oil droplets into the surrounding aqueous environment. Depending on its solubility and the volume of the aqueous environment, the drug may dissolve or precipitate, which may lead to unreliable results. The method utilizing the type II apparatus can be used in the study of the release of substances from nanoemulsions when the concentration of the substance in the formulation exceeds its water solubility. Moreover, the large volume of the dissolution medium can help overcome the difficulties in maintaining sink conditions for poorly soluble drugs. In vitro drug release studies using membrane-free diffusion methods have also been described. However, because of the direct contact of the tested systems with the dissolution medium, their possible aggregation and/or disintegration in the dissolution medium should be assessed. In vitro drug release experiments are typically conducted under sink conditions which can be achieved with a relatively large volume of dissolution medium (i.e., from 40 to 200 mL). It can be expected that the small volume of the dissolution medium more accurately reflects the in vivo conditions since the average amount of tear fluid produced in the precorneal area during the 24 h period is 2 mL. However, in an in vitro drug release study from contact lenses, it has been shown that small volumes of dissolution medium are not suitable for in vitro testing or do not reflect the precorneal environment.In order to minimize the effects of the unstirred aqueous layer, in vitro drug release experiments are performed at different agitation rates; for example, from 20 to 100 rpm for dialysis methods using a paddle dissolution apparatus, to 150 and 600 rpm for simple dialysis and Franz diffusion cell methods. Drug release studies are also performed using vertical Franz diffusion cells with an effective area of 1.13 cm2 into simulated tear fluid at pH 7.4. The nanoemulsion (1 mL) is deposited onto the previously soaked dialysis membrane which separates the donor and acceptor chambers, taking samples at regular intervals and replacing them with the same volume of fresh medium.It should be stressed that sink conditions in the eye can be maintained if the drug clearance is high. However, the total clearance mechanism (including lacrimal turnover and absorption by the conjunctiva) is complex and difficult to simulate in in vitro studies. In physiological conditions the human eye contains a tear volume that ranges from 6.2 to 30.0 ?L and the tear flow rate assumes values between 0.9 and 2.1 ?L/min. In order to predict the drug release kinetics in the eye in a more reliable way , it is crucial to develop microfluidic models that mimic the hydrodynamic conditions of the eye as accurate as possible.
- Glaucoma disease Glaucoma is a group of eye conditions that damage theoptic nerve, vital for transmitting visual information to thebrain. Typically, the damage is due to elevated intraocularpressure (IOP), which is often caused by an imbalancebetween the production and drainage of the eye fluid(aqueous humor). The build-up of fluid can result fromissues with the outflow of intraocular fluid from the iristhe coloured part of the eye). This condition can resut in gradual vision loss and, if left untreated, can lead toblindness.12Various factors, such as irritationorblockageof the drainage pathway, can lead to inadequate fluidoutflow. After cataracts, glaucoma is the second-largestcause of visual loss. Overcoming barriers to effectivelydeliver drugs to the eyes involves tackling variouschallenges. These include tight junctions in the cornealepithelium, the natural blinking reflex, increased tearproduction, difficulties in absorption through the corneaand conjunctiva, and limited capacity of the conjunctivalsac.Glaucoma is a neurodegenerative disease, that affects theretinal nerve fibers and opticnervehead, and is a leadingcause of global visual impairment. Projections suggestthat by 2040,approximately 111.8 million individualsworldwide will be affected. Glaucoma vision loss sisaccompanied by stiletto field losses that advance fromone retinotopic region to the next. The responsivenessto IOP, whichmeasures the generation and outflow ofaqueous fluid in the anterior eye, is associated with these.impairments.Two types of glaucoma can be considered as major types,(a) open-angle glaucoma, and (b) closed-angle glaucoma.The first type which is primary open-angle glaucoma(POAG) is the most common (3). A critical pathologicalrisk factor for POAG involves intraocular pressure levels,which are produced within the anterior chamber of theeye due to resistance in draining aqueous humorfromthe trabecular meshwork and the inner wall of Schlemm’scanal.8 Generally, the breadth of the angle between thecornea and iris permits normal aqueous fluid outflowfrom the anterior chamber to the drainage channels ofthe trabecular meshwork. Finally, the issue with fluidoutflow was caused by the malfunction of the trabecularmeshwork, which becomes blocked by cell debris,including inflammatory or blood cells. The most prevalenttype of glaucoma is primary open-angle glaucoma (orPOAG), which is linked to increased resistance in theaqueous outflow channels. 9 Often, there are no immediateor acute signs or symptoms and no discomfort13. The onlysymptoms that worsen over time are narrowing of thevisual field and alterations in the optics nerve. 10 Secondary,closed-angle glaucoma, characterized by the narrowingor occlusion of the outflow route, hinders the drainage of aqueous fluid, potentially leading to sudden spikes inIOP and significant ocular discomfort. Another typeof glaucoma is congenital glaucoma, which manifestsin babies with symptoms such as excessive tearing, lightsensitivity, a fear of light, and eyelid retraction. Thesesigns are often accompanied by a noticeable enlargementand cloudiness of the cornea.The study of glaucomaand its consequences has been the focus of numerousinvestigations. illustrates a comparison between eyes with normal and increased IOP, whereas the secondcomparison, shown in , distinguishes between open-angle and closed-angle glaucoma.Glaucomacan also be considered as a set of ocularneuropathic conditions that together account for theworld’s leading cause of irreversible blindness, whereblindness is caused by the degeneration of neural tissuesin the optic projection from the retina to the brain. 14Glaucoma is becoming more common as the populationages; by 2040, nearly 112 million people will be affectedworldwide. Traditional ophthalmic solutions, employed over anextended period, have limitations in terms of therapeuticeffectiveness. Challenges include drug elimination
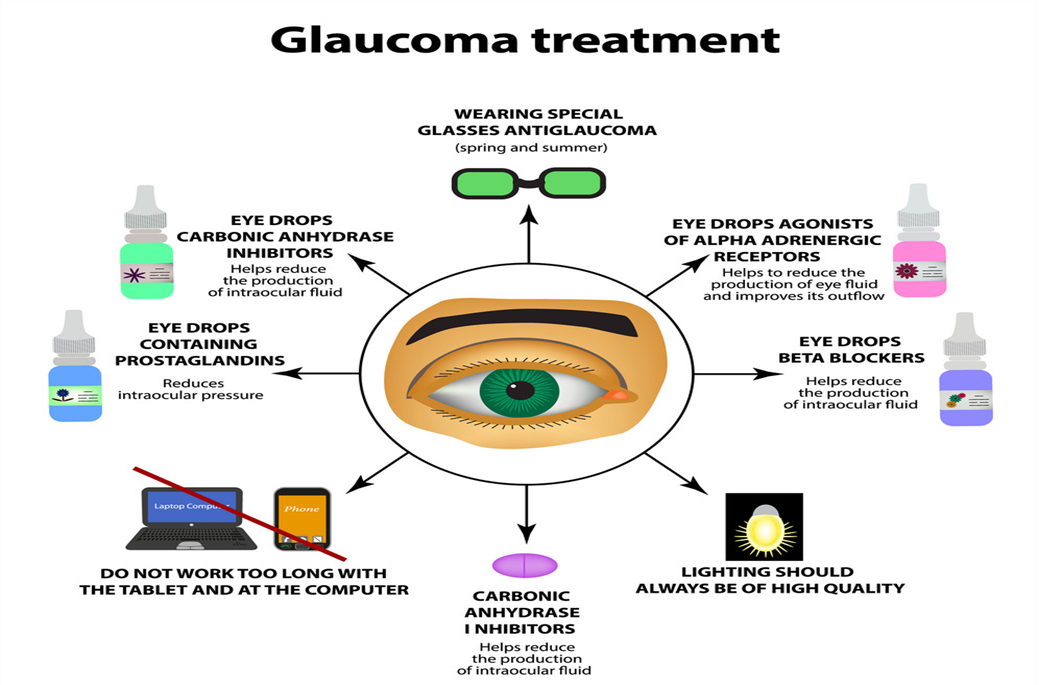
- conventional glaucoma treatment and its limitation
The current objective of glaucoma treatment are to prevent damage to the optic nerve, preserve the patient s visual field, and mamitaintheire quality of life by minimizing the side effect of medication of the several factor that might increase the risk of glaucoma development, IOP is considered the most crucial and omly practicable risk factor of glaucoma IOP reduction inhibits the progression of glaucoma Threfore, it is essential to prioritize IOP the therpy consists of applying antiglucoma eye drops directly to the affected area, followed by the administration of oral drug, lasur treatment, and surgery if necessary. The treatment of glaucoma can be categorized into medical therpy, lasaretherapy and surgery 15Topical eye drop of antiglucoma drugs effectively reduced the IOP and several therapeutic agent with specific mechanism are used for ex, cholinergic agonists stimulnate the contraction of the ciliry muscle, leading to a more spherical shape of the lense and an increase muscle, leading to a more spherical shape of the lenses and an increase in theire focusing ability, and also contracted the cells of the lences and increses in theire focusing ability, and also conttraced the cell of trabecular meshwork [TM].

16The surface of the eye after a single blink of eye, and almost all administered formulation is cleared from the eye surface within 15-25 min allowing a short duration of about 5-7 min for absorpation of administered drug eventually around 5% of topically administered formulation may overawed the barrires and reached is required, which lesds to patimt incompliance and early termination of medication these ocular barrirs also play a role in the fluctuating threpeutic effect that occurs before and after each appalication of the eye drops the variation in medication concentration over time might cause fluctuations in IOP at various times intervals of the day which is likely to contribute to the development of glaucoma Sometime ,medical therpy may not effectively reduce the IOP to the desired levels, despite the use of the most effectively medications for management of glaucoma, the use disease may still progress and lead to the degradation of the optic nerve in such cause,trabeculoplasty may be investigated as a treatment option to decrease IOP in patint with open-angle glaucoma argon laser trabecuoplasty (ALT) involve targeting the TM cells carrying pigment with laser triggering coagulative necrosis as well as thermal damage which induce the contraction of the TM hence increasing the drainage capacity of the aqueushumor the argon lasae harms extends beyond the target region containing melanin protin. The use of ALT might result in significant advers effects, including peripheral anterior anterior synechiae, uveitis and temporary icreases in IOP conversely Q-swithched Nd: YAG laser with double frequency is used for the purpose of selective laser trabecuplastry (SLT) decreases IOP by various mechanism, including the stimulation of and extracellular matrix synthesis and turnover, displacement of cell extraction matrix (ECM) synthesis
The idea of nanocarrires was first introduced in 1980s; however the word nanomedicine was first officially definds by european science foundation (ESF) in 2003.17Finally, in 2010 the precise definition of nanomedicine was established as the extentesive monitoring regulate construction repaire, defence and enhacement of biological system of human at molecular level with help of engineered nanostructures and nanodevices operating single-cell level with the ultimate goal of achievices enhanced therapeutic benefits nanomedicines,a branch of nanotechnology, playa significant role in the diagnosis and treatment of various disease including ocular diseases by greatly enhacing the efficacy of therepics. Nanomedicine has the capacity to encapsulate a wide range of therapeutic –agent. 18The enacasulation of drug molecule in nanomedicine proteck them from degradation and also inhance the targeting ability via surface modification significant effectively to anteriorsegment of eye. Chitosan (CH), hydroxypropyl methylecellulose(HPMC), gellangelatin, hyaluronic acid(HA), and carboxmethlcellulose are often used as a mucoadhesive agets in the formulation nanomedicines to extend the retention time on ocular surface and may show resistance to ocular clearance as a result of the movement of blinling and the impulsive production of tears. Furthermore, the outermost layer of the cornea, know as the superficial corneal epithelium, is coated with a mucin membrane that carries a negative charge thise creates an ideal surface for attachaing nanocarriers that have a positive charge
Case studies of nanomedicine for glaucoma treatment
|
Drug
|
Dosage
|
Application/Advantages
|
|
Timolol
|
Contact lenses
|
Bioavailability enhancement
|
|
Dorzolamide
|
Drops
|
Increased corneal penetration
Ability, prolonged drug action
Time with sustained drug
|
|
Pilocarpine
|
Drops
|
Poolonged drug action time
With sustained drug relese
|
|
Travoprot
|
Drops
|
Increased corneal penetration
Abiity.
|
|
Timolol
|
Drops
|
Enhanced corneal permeati-
-on ability.
|
|
Noisome
|
Gels
|
Prolonged drug action time
With sustained drug relese
|
|
Timolol
|
Gel
|
Prolonged drug action time
With sustained drug relese
|
-
Composition of the nanoemulsions administered to eye
Ophthalmic o/w nanoemulsions can generally be considered as dispersions of oily droplets in an aqueous environment. Therefore, these formulations require careful selection of the composition of both the oily phase (i.e., the use of nontoxic, nonirritating, pharmaceutically approved oils) as well as the composition of the aqueous medium . Similarly to the ophthalmic drops the need for isotonicity of the ocular nanoemulsions, the desired pH, a certain buffering capacity, the addition of preservatives (antimicrobial agents), viscosity modifiers, and antioxidants calls for careful consideration19. Furthermore, in order to obtain nanosize uniform droplets of oils, an extensive optimization of the composition as well as the concentration of surfactants and cosurfactants in the formulation are required. The complexity of ophthalmic nanoemulsion compositions demands comprehensive knowledge and experience in pharmaceutical formulations so stable products of pharmaceutical quality couldbe designed. In this section we provide detailed characteristics of the most frequently used components of ophthalmic nanoemulsions including oils, emulsifiers, surfactants, and cosurfactants as well as additives used to modify their pharmaceutical properties (e.g., tonicity, viscosity, or pH of the formulation
Ophthalmic nanoemulsions contain from 5 to 20 wt % of oil/lipid as the dispersed phase. The selection of the lipid phase is an important aspect in the design of nanoemulsions, as API is dissolved in an oil prior to the dispersion in an aqueous phase. The selection of the oil phase for nanoemulsion formulation is frequently based on the solubility of API in different oils.9 In addition, the oil used in the formulation needs to be well tolerated and compatible with the other excipients included in the nanoemulsion. The following compounds are frequently used to prepare ophthalmic nanoemulsions vegetable oils, glycerides, medium-chain triglycerides, long-chain unsaturated fatty acids, and polyalcoholic esters of medium-chain fatty acids20. Vegetable oils administered topically to the eye include: soybean oil, castor oil, peanut oil, olive oil, jojoba oil, and Babchi seed oil.Medium-chain triglycerides such as Miglyol 812, Captex 355, 200 or 8000, Witepsol, and Labrafac as well as long-chain unsaturated fatty acids (oleic and octanoic acids) are also used as an inner phase of nanoemulsions. From the group of polyalcoholic medium-chain and long-chain fatty acid esters, the following are used: ethyl oleate, isopropyl myristate and isopropyl palmitate. Triacetin and vitamin E are also mentioned among the ingredients of the nanoemulsion applied to the eye, and additionally, these two compounds in ophthalmic formulations can act as humectants and antioxidants.
- Applications of ophthalmic nanoemulsion drug delivery systems
21The form of nanoemulsion for ophthalmic drug delivery system for treating several eye diseases like dry eye syndrome, glauco.Several drugs are formulated in ma, etc . A combination of oil in water type emulsions are being generated usually for improved topical lipophilic drug delivery to the eye. Lipophilic drug is usually having increased absorptivity compared to lipophobic. The bioavailability is enhanced, and the patient compliance is found to be better when nanoemulsion are topical
Nanoemulsinsare extensively used in pharmaceutical dosage forms for delivery of drugs to eye, which proposes several benefits such asNanoemulsi delivery of medications, biological or analytical agents. The most significant application of ophthalmic nanoemulsion is longer residence time in the eye cavity and larger bioavailability in comparison with the conventional drug delivery systems like eye drop solutions and suspensions. Ophthalmic nanoemulsions may also deliver the drugs, which are prone to hydrolysis and oxidation overall all ophthalmic nanoemulsion preparation by be considered as active safe and with superior bioavilability it is predictable that additional research and development will be conduced out in the future concerning ophalmic nanoemulsion.
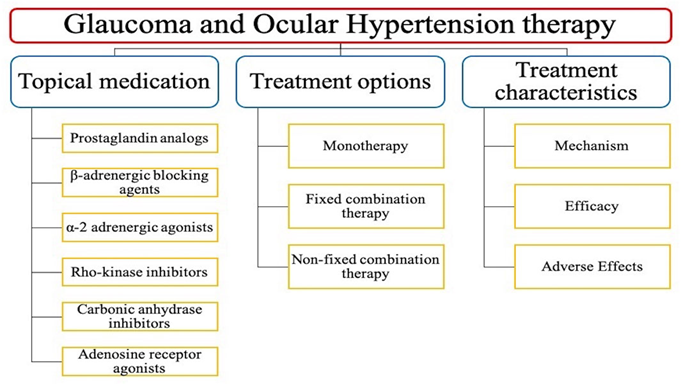
Classification of glaucoma treatment
- Glaucoma medication delivered via nanoemulsions
1. Timolol maleate; A beta-blocker used to reduce intraocular pressure.
2. Pilocarpine; A muscarinic recptor agonist used to reduce intraocular pressure.
3. Latanoprost;A prostaglandin analogusedto reduce intraocular pressure.
4. Brimonidine; An alpha-2 adrenergic agonist used to reduce intraocular pressure.
Timolot maleate
The maleate salt form of timolol, a propanolamine derivative and a non-selective beta-adrenergic antagonist with antihypertensive property. 22Timolol competitively binds to beta-1-adrenergic receptors in the heart and vascular smooth muscle and beta-2-receptors in the bronchial and vascular smooth muscle, resulting in a decrease in beta-adrenergic stimulation. Beta-1-receptor blockade results in a decrease in resting and exercise heart rate and cardiac output, a decrease in both systolic and diastolic blood pressure, and, possibly, a reduction in reflex orthostatic hypotension. Beta-2-blockade results in an increase in peripheral vascular resistance. The ultimate results include vasodilation, and negative chronotropic and inotropic effects. In addition, timolol reduces intra-ocular pressure possibly by decreasing aqueous humor production by reduction of blood flow to the ciliary processes and cAMP synthesis.Timolol is used in the treatment of ocular hypertension and glaucoma Timolol is a beta blocker. It works by decreasing the production of aqueous humour (fluid in the eye), thereby lowering the increased eye pressure
Machanishm Ction Of Timolol Maleate In Gluacoma:
Open-Angle Glaucoma
The exact mechanism of action by which timolol reduces the intraocular pressure in patients with open-angle glaucoma is unknown. 21Timolol is thought to inhibit ?-receptors on the ciliary epithelium, which normally functions to increase the production of aqueous humor. Some researchers have proposed that by inhibiting these receptors, timolol reduces aqueous humor production and, therefore, reduces intraocular pressure.Researchers have also studied non-adrenergic pathways of timolol to reduce intraocular pressure.
Ocular Hypertension
Timolol's mechanism of action in reducing intraocular pressure in patients with ocular hypertension is unknown22. Still, it appears similar to its mechanism in lowering intraocular pressure for patients with open-angle glaucoma.
Infantile Hemangioma
Timolol is thought to antagonize ?-adrenergic receptors, causing multiple processes, including vasoconstriction, apoptosis stimulation, and angiogenesis inhibition.
Hypertension
The sympathetic nervous system is an essential component of blood pressure regulation. Usually, ?-1 and ?-2 receptors are activated by endogenous catecholamines. 23These activated receptors stimulate their associated G-protein, activating adenylyl cyclase and increasing cyclic-AMP (cAMP) levels.This secondary messenger initiates a cascade of reactions in the body, including vasoconstriction and elevated blood pressure. Nonselective ?-blockers (eg, timolol) block interactions between endogenous catecholamines and prevent the G-protein cascade from occurring, reducing sympathetic tone and decreasing blood pressure.
Myocardial Infarction
There are many mechanisms by which ?-blockers can reduce morbidity and mortality in patients after myocardial infarction. For example, ?-blockers can reduce myocardial oxygen demand and relieve ischemic chest pain. By blocking the sympathetic receptors in the heart, timolol decreases heart rate. This induced bradycardia prolongs diastole, allowing for increased perfusion of the heart.Beta-blockers can inhibit platelet aggregation and thromboxane synthesis and reduce the rate of atherosclerosis and thromboembolism. They also inhibit cardiac remodeling after myocardial infarction.
Migraine Prophylaxis
The exact mechanism of timolol for migraine prevention is unknown. One proposed mechanism involves the antagonism of ?-adrenergic receptors, which decreases the synthesis and release of norepinephrine, a key intermediate in the pathophysiology of migraines. Another mechanism that could explain timolol's migraine prophylactic properties is based on the ability of ?-blockers to regulate the neuronal firing of periaqueductal gray matter using gamma-aminobutyric acid (GABA)24,25. Timolol also appears to play a role in regulating the serotonergic system by inhibiting serotonin (5-HT), another important mediator in the pathophysiologic pathway of migraines26. This modulation of serotonin's effects seems to contribute to the ability of ?-blockers to reduce the sensitivity of the auditory system, reducing the frequency of migraine attacks. There is also a hypothesis that ?-blockers play a significant role in reducing the excitability of the visual system in patients with migraines. Beta-blockers such as timolol are also thought to reduce the spread of signals through the brain, including the cortical spread and the excitability of the ventroposteromedial thalamic nucleus.
Atrial Fibrillation
The autonomic nervous system plays a significant role in developing atrial fibrillation. Aberrant sympathetic tone can stimulate myocyte contraction and promote irregular rhythms in susceptible patients. Beta-blockers help maintain the heart's regular rhythm by decreasing the autonomic tone and, therefore, decreasing sympathetic stimulation of the cardiac myocytes.
CONCLUSION
The ophthalmic drug market has significantly grown in recent years globally. Between 2015 and 2018 there was an 800% increase in approvals of new ophthalmic drugs including topical treatments.118?120 Although manufacturers are increasingly investing in research and development of innovative, noninvasive ophthalmic preparations, there is still a significant unmet need for the availability of multiactive ophthalmic drug formulations, including combination therapy products. Formulation of complex innovative drug delivery systems, such as ophthalmic nanoemulsions, requires extensive understanding of pharmaceutical aspects related to the selection of auxiliary substances and quality control methods that guarantee the desired properties, stability, and tolerance of the formulation during shelf life and after its application. Furthermore, from a formulation scientists’ perspective, it is of great importance to select robust optimizable technological processes enabling efficient manufacturing of ocular nanoemulsions on an industrial scale. This also includes formulation of sterile products as well as selection of packing and dosing devices. Ophthalmic nanoemulsions formulations require optimization of both composition and manufacturing methods. The optimization process involves several critical nanoemulsion properties, for example, particle size and its distribution,stability, contact time at the application site, and tolerance.The available research indicates that size of oil phase droplets is strongly affected by the nanoemulsion composition, manufacturing technique, and preparation conditions. Therefore, there is a need for continuous research enabling tounderstand the interplay between the composition of nanoemulsions, their preparation techniques, and properties of the formulation. Furthermore, successful implementation of the nanoemulsion-based formulations into medical practice requires a comprehensive evaluation of their quality using well-defined, standardized analytical methods, and research protocols. Despite the continued interest in nanoemulsions formulation with low-energy methods, a growing share of the use of high-energy methods is observed
REFERENCE
- Jafari, S.; McClements, D. J. Nanoemulsions: Formulation, Applications, and Characterization; Academic Press, 2018.
- Lim, C.; Kim, D.-w.; Sim, T.; Hoang, N. H.; Lee, J. W.; Lee, E. S.; Youn, Y. S.; Oh, K. T. Preparation and Characterization of a Lutein Loading Nanoemulsion System for Ophthalmic Eye Drops. J. Drug Delivery Sci. Technol. 2016, DOI: 10.1016/j.jddst.2016.10.009
- Ammar, H. O.; Salama, H. A.; Ghorab, M.; Mahmoud, A. A. Nanoemulsion as a Potential Ophthalmic Delivery System for Dorzolamide Hydrochloride. AAPS PharmSciTech 2009, 10 (3), DOI: 10.1208/s12249-009-9268
- Morsi, N. M.; Mohamed, M. I.; Refai, H.; El Sorogy, H. M. Nanoemulsion as a Novel Ophthalmic Delivery System for Acetazolamide. Int. J. Pharm. Pharm. Sci. 2014, 6 (11
- El Qidra, R. K. A Novel Ophthalmic Pharmaceutical Nano Emulsion: Methods of Preparations, Characterizations, and Applications. Am. J. PharmTech Res. 2015, 5
- Ranadive F, Surti AZ, Patel H. Predicting Glaucoma Diagnosis Using AI. Intelligent Systems Reference Library 2022; 206: 51-76. https://doi.org/10.1007/978-3-030-76
- Siggers JH, Ethier CR. Fluid Mechanics of the Eye. Annu Rev Fluid Mech 2011; 44: 347-72. https://doi.org/10.1146/ANNUREVFLUID-120710-101058.
- Mayo Clinic Guide to Better V. Glaucoma - Symptoms and causes - Mayo Clinic. 2020; Available from: https://www.mayoclinic.org/ diseases-conditions/glaucoma/symptoms-causes/syc-20372839.
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014; 121: 2081-90. https://doi.org/10.1016/J. OPHTHA.2014.05.013.
- Lin Y, Jiang B, Cai Y, Luo W, Zhu X, Lin Q, et al. The Global Burden of Glaucoma: Findings from the Global Burden of Disease 2019 Study and Predictions by Bayesian Age–Period–Cohort Analysis. J Clin Med 2023; 12: 1828-. https://doi.org/10.3390/JCM12051828.
- Qing G, Zhang S, Wang B, Wang N. Functional MRI Signal Changes in Primary Visual Cortex Corresponding to the Central Normal Visual Field of Patients with Primary Open-Angle Glaucoma. Invest Ophthalmol Vis Sci 2010; 51: 4627-34. https:// doi.org/10.1167/IOVS.09-4834.
- Dillinger AE, Guter M, Froemel F, Weber GR, Perkumas K, Stamer WD, et al. Intracameral Delivery of Layer-by-Layer Coated siRNA Nanoparticles for Glaucoma Therapy. Small 2018; 14: 1803239-. https://doi.org/10.1002/SMLL.201803239.
- Calkins DJ. Adaptive responses to neurodegenerative stress in glaucoma. Prog Retin Eye Res 2021; 84: 100953-. https://doi. org/10.1016/J.PRETEYERES.2021.100953.
- Tezel G. Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog Retin Eye Res 2021; 100998. https://doi.org/10.1016/J. PRETEYERES.2021.100998.
- Khazaeni B, Khazaeni L. Acute Closed Angle Glaucoma. StatPearls [serial on the Internet]. 2017: Available from: http://europepmc. org/books/NBK430857.
- Prum Jr BE, Herndon Jr LW, Moroi SE, Mansberger Mph SL, Stein Ms JD, Lim MC, et al. Primary Angle Closure Preferred Practice Pattern® Guidelines. Ophthalmology 2016; 123: P1-P40. https://doi. org/10.1016/j.ophtha.2015.10.04
- Patel, A. Ocular Drug Delivery Systems: An Overview. World J. Pharmacol. 2013, 2 (2), 47.
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H. M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A. K. A Comprehensive Insight on Ocular Pharmacokinetics. Drug Delivery Transl. Res. 2016, 6, 735?754.
- Lang, J. C. Ocular Drug Delivery Conventional Ocular Formulations. Adv. Drug Delivery Rev. 1995, 16, 39?43.
- Pal Kaur, I.; Kanwar, M. Ocular Preparations: The Formulation Approach. Drug Dev. Ind. Pharm. 2002, 28 (5), 473?493.
- Le Bourlais, C.; Acar, L.; Zia, H.; Sado, P. A.; Needham, T.; Leverge, R. Ophthalmic Drug Delivery Systems - Recent Advances. Prog. Retinal Eye Res. 1998, 17, 33?58.
- Järvinen, K.; Järvinen, T.; Urtti, A. Ocular Absorption Following Topical Delivery. Adv. Drug Delivery Rev. 1995, 16, 3?19.
- Gorantla, S.; Rapalli, V. K.; Waghule, T.; Singh, P. P.; Dubey, S. K.; Saha, R. N.; Singhvi, G. Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 2020, 10, 27835?27855.
- Souto, E. B.; Dias-Ferreira, J.; López-Machado, A.; Ettcheto, M.; Cano, A.; Espuny, A. C.; Espina, M.; Garcia, M. L.; Sánchez-López, E. Advanced Formulation Approaches for Ocular Drug Delivery: Stateof-the-Art and Recent Patents. Pharmaceutics 2019, 11, 460.
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S. Successfully Improving Ocular Drug Delivery Using the Cationic Nanoemulsion, Novasorb. J. Drug Delivery 2012, 2012, 1?16.
- Khiev, D.; Mohamed, Z. A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Lobo, G. P.; Valapala, M.; Kerur, N.; Passaglia, C. L.; Mohapatra, S. S.; Biswal, M. R. Emerging Nano-Formulations and Nanomedicines Applications for Ocular Drug Delivery. Nanomaterials 2021, 11 (1), 173


 Avinash Gite*
Avinash Gite*
 Nikam.H.M
Nikam.H.M





 10.5281/zenodo.14634480
10.5281/zenodo.14634480