Abstract
Microspheres, a pivotal advancement in multiarticulate drug delivery systems, represent a promising approach to achieving controlled, sustained, and targeted drug release. These spherical carriers, typically ranging from 1 to 1000 µm in diameter, are designed to improve therapeutic efficacy, enhance bioavailability, and reduce side effects. The formulation of microspheres involves meticulous consideration of factors such as polymer selection, drug loading, encapsulation efficiency, and particle size, each of which significantly influences the performance of the delivery system. Various methods, including solvent evaporation, spray drying, ionic gelation, and solvent diffusion, are employed to formulate microspheres, each with distinct advantages and limitations. Characterization techniques such as scanning electron microscopy (SEM), dynamic light scattering (DLS), and Fourier transform infrared spectroscopy (FTIR) are utilized to ensure the desired properties and stability of the microspheres. The versatility of microspheres extends to diverse applications, including controlled and targeted drug delivery, vaccine delivery, cancer therapy, and gene therapy. Despite these advantages, challenges such as scalability, stability, and regulatory approval persist. Advances in polymer science, nanotechnology, and personalized medicine are driving innovation in this field, paving the way for the development of stimuli-responsive and smart microspheres capable of precise therapeutic interventions. This review delves into the formulation strategies, optimization parameters, characterization techniques, and applications of microspheres as drug delivery systems. It also highlights current challenges and explores future prospects, underscoring the potential of microspheres to revolutionize modern therapeutics and improve patient outcomes.
Keywords
Microspheres, Multiarticulate, Systems, Therapeutic, Enhance bioavailability treatment, Physicochemical
Introduction
Microspheres are emerging as a cornerstone in the field of multiarticulate drug delivery systems, offering unparalleled versatility and efficacy. Unlike traditional monolithic delivery systems, microspheres consist of numerous discrete units, enabling uniform distribution of the drug and minimizing the risks associated with dose dumping. This multiarticulate nature ensures a more predictable and consistent release profile, making microspheres an attractive option for controlled and sustained drug delivery applications [1]. The concept of multiarticulate drug delivery is driven by the need to overcome the limitations of single-unit systems, such as uneven drug distribution and variability in therapeutic response. Microspheres, owing to their small size and high surface area-to-volume ratio, enable precise control over drug release kinetics and enhance the bioavailability of poorly soluble drugs. Their ability to encapsulate a wide range of active pharmaceutical ingredients, from small molecules to biologics, further underscores their adaptability [2,3].
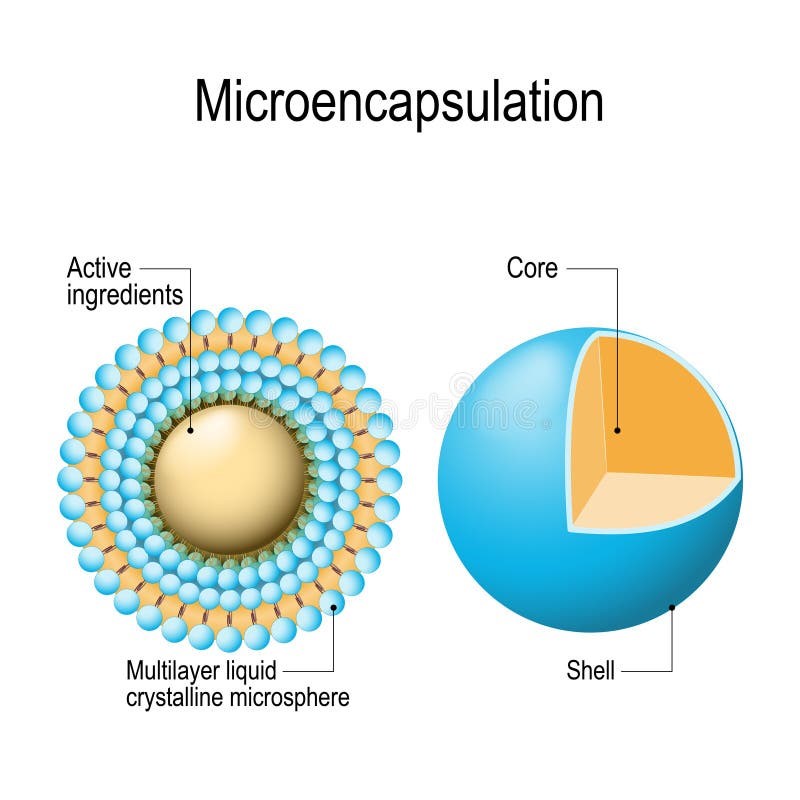
Figure 1: Microspheres
Microspheres can be fabricated using natural or synthetic polymers, allowing researchers to tailor their properties for specific applications. Biodegradable polymers such as polylactic acid (PLA) and polylactic-co-glycolic acid (PLGA) are particularly advantageous for sustained-release formulations, as they degrade into non-toxic byproducts [3,4]. Non-biodegradable polymers, on the other hand, offer prolonged release durations, making them suitable for chronic conditions. The multiarticulate nature of microspheres not only reduces the risk of dose dumping but also facilitates administration through diverse routes, including oral, parenteral, and pulmonary. This flexibility makes them ideal for addressing various therapeutic challenges, such as improving patient compliance and targeting specific tissues or organs. Additionally, microspheres provide a platform for the development of advanced drug delivery systems, including stimuli-responsive and smart delivery mechanisms [5].
Despite their numerous advantages, the development of microsphere-based drug delivery systems is not without challenges. Issues such as scalability, reproducibility, and regulatory hurdles must be addressed to ensure their successful translation from research to clinical practice. This review focuses on the formulation and optimization of microspheres, providing insights into their potential to revolutionize drug delivery and improve therapeutic outcomes [6]. The design and development of advanced drug delivery systems have become a cornerstone in modern pharmaceutical science. Traditional drug delivery methods, while effective in certain cases, often fall short in achieving optimal therapeutic outcomes due to issues such as poor bioavailability, frequent dosing requirements, and undesirable side effects. To address these challenges, researchers have turned to multiarticulate drug delivery systems, among which microspheres stand out for their versatility and efficiency. Microspheres are defined as spherical particles ranging from 1 to 1000 µm, typically composed of natural or synthetic polymers [7]. These systems are designed to encapsulate active pharmaceutical ingredients (APIs), offering controlled release, targeted delivery, and protection of sensitive drugs from degradation. The multiarticulate nature of microspheres ensures uniform distribution of the drug, reducing variability in therapeutic effects and enhancing patient compliance [8,9]. This review provides a detailed overview of the formulation strategies, optimization approaches, and applications of microspheres, emphasizing their potential to revolutionize drug delivery systems. It also discusses current challenges and future directions in the field, highlighting the need for continued innovation to overcome existing limitations and unlock the full potential of this technology.
Types of Microspheres
Microspheres can be classified into various types based on their composition, biodegradability, and functional characteristics. A detailed understanding of these types is essential for tailoring microsphere formulations to specific therapeutic needs [10].
Biodegradable Microspheres
Biodegradable microspheres are formulated using polymers that degrade into non-toxic by-products, such as carbon dioxide and water, within the body. These microspheres are particularly suitable for sustained-release drug delivery applications, as they eliminate the need for surgical removal post-therapy.
- Polylactic Acid (PLA): A hydrophobic, slow-degrading polymer often used for long-term drug release.
- Polyglycolic Acid (PGA): Faster degrading than PLA, suitable for short-term therapeutic effects.
- Polylactic-co-Glycolic Acid (PLGA): Combines the properties of PLA and PGA, allowing customization of degradation rates.
- Natural Polymers: Examples include gelatine, alginate and chitosan, which are biocompatible and biodegradable, making them ideal for biomedical applications [11,12].
Non-Biodegradable Microspheres
These microspheres are designed for applications requiring extended drug release without polymer degradation. They are generally used for chronic conditions and require surgical removal after the therapy is completed.
- Polymethyl Methacrylate (PMMA): Widely used for its stability and compatibility.
- Ethyl Cellulose: Provides sustained release of drugs, commonly used in oral formulations.
- Polyvinyl Alcohol (PVA): Known for its robust mechanical properties and minimal interaction with the encapsulated drug [13].
Functionalized Microspheres
Functionalized microspheres are designed to respond to specific stimuli, offering enhanced control over drug release.
- Magnetic Microspheres: Incorporate magnetic materials, enabling drug targeting using an external magnetic field. They are commonly used in cancer therapy.
- pH-Sensitive Microspheres: Designed to release drugs at specific pH levels, suitable for targeting the gastrointestinal tract.
- Thermo-Responsive Microspheres: Release drugs in response to temperature changes, ideal for hyperthermia-induced therapies [14].
Polymeric Microspheres
Polymeric microspheres are categorized based on the type of polymer used:
- Natural Polymers: Include albumin, gelatine, and collagen, which are biocompatible but may have stability issues.
- Synthetic Polymers: Such as PLGA, PEG and PMMA, provide excellent control over drug release profiles and stability [15].
Ingredients of Microspheres
Polymers:
In microsphere formulation, most commonly biodegradable and non-biodegradable variety of polymers are used by researchers. The polymers which are used in microspheres preparation are classifies into different types as Natural and Synthetic. Before choosing the polymer for the microsphere formulation, we need to consider a few parameters such as nontoxicity, biocompatibility, biodegradability, and easy availability of polymers. It should be biocompatible, biodegradable, non-toxic, and easily available. These polymers which pass all parameters for their selection have many advantages like they increase the residence time of the drug in the body because of which we get better bioavailability of drug compared to conventional drug delivery system. Examples of natural polymers include Albumin, Collagen, and Gelatin which are proteins while Agarose, Carrageenan, Chitosan, and Starch are carbohydrates whereas Poly (acryl) Dextran, Poly Starch, and DEAE Cellulose are chemically modified carbohydrates and Sodium Alginate, cellulose ether, xanthan gum, Scheroglucan, Gum Arabica, Tamarind seed polysaccharide, Beeswax, carnauba wax, Chitin, and Corn protein (Zien) form the other class. Examples of synthetic polymers fall under three category where Lactides, Glycolides and their copolymers, polyanhydrides, poly alkyl cyanoacrylates are biodegradable in nature while Glycidyl methacrylate, Acrolein, Epoxy polymers, and polymethyl methacrylate are non-biodegradable and finally, polysebacic anhydrides, Poly Esters/Poly Lactides, poly orthoesters, polycarbonates, polylactic glycolic acid (PLGA), polycaprolactones, polyphosphazenes, ethylcellulose, Eudragit L100, Eudragit S100, HPMC, Eudragit RS100, and Eudragit RL100 form the other class [16,17].
Surfactant
In microsphere formation surfactant play an important role during emulsification and extrusion process. Surfactants play an important role by lowering the interfacial tension between hydrophilic and hydrophobic molecules, because of which stable emulsion is formed. Use of surfactant leads to the formation of discrete microspheres by preventing the emulsion droplets from coalescing. Hydrophilic– lipophilic balance (HLB) indicator is used for selection of proper emulsifier. Hydrophilic surfactants have HLB value in the range of 8–18 and are used for oil in water emulsion while emulsifiers with HLB value in the range of 3.5–6 are known as lipophilic surfactants. The particle size of microspheres is decreased by increasing the concentration of surfactant because of which smaller size and size distribution of microspheres were formed. Examples are Sodium Laureth Sulfate, Sodium dodecyl sulfate as anionic surfactants and Polysorbate 80, Tween 40, Tween 20, Span 85, Span 80, Span 20, Poloxamer188, Brij58, Poly Glycerol Polyricinoleate, and Sorbitan as non-ionic surfactants [18,19].
Oil
Particle size, size distribution, and uniformity of microspheres are affected by the ratio of viscosity of the oil phase to the viscosity of the water phase, for example, it is reported that the particle size of microspheres is more which were prepared using olive oil compared to microspheres which were prepared using liquid paraffin as the viscosity of olive oil is higher compared to liquid paraffin oil. There are various types of oils which are used in the fabrication of microspheres during emulsification/gelation method. Examples are liquid paraffin, soya bean oil, olive oil, sunflower oil, castor oil, groundnut oil, rapeseed oil, and rapeseed methyl esters [20].
Crosslinkers
Most commonly used crosslinkers for microspheres preparation are Ca2+, Sr2+, and Ba2+ ions. However, Sr2+ and Ba2+ ions are mildly toxic and Ca2+ ions are non-toxic because of which Ca2+ ions are widely used crosslinkers for preparation of microspheres. At low concentration of Ca2+ ions agglomeration of microspheres takes place. By increasing Ca2+ ions concentration entrapment efficiency of microsphere slightly increases. However, after optimum concentration of cross-linker if more crosslinker is added the entrapment efficiency decreases due to overloading of crosslinker. Examples are glutaraldehyde, sulfuric acid and calcium carbonate [21-23].
Solvents
Solvents mostly used when microspheres are prepared using a solvent evaporation method. Examples are Chloroform, Dichloromethane (DCM), Ethanol, Acetonitrile, Polyvinyl alcohol (PVA), Methylene chloride, and Methanol [24].
Formulation Methods of Microspheres
The formulation of microspheres involves selecting appropriate polymers, drugs, and techniques to achieve the desired characteristics. The commonly used methods are:
Emulsion-Solvent Evaporation Method
This method involves dissolving the drug and polymer in an organic solvent to form a homogenous solution. The solution is then emulsified in an aqueous phase containing a stabilizer under vigorous stirring, resulting in the formation of droplets. As the organic solvent evaporates, the polymer solidifies around the drug, forming microspheres. Critical factors such as the type of solvent, stabilizer concentration, and stirring speed play a crucial role in determining the size and encapsulation efficiency of the microspheres. This method is widely used due to its simplicity and scalability, though the use of organic solvents may pose environmental and safety concerns [25].
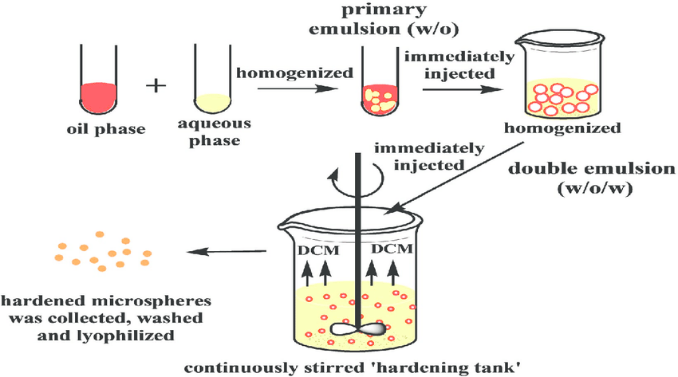
Figure 2: Emulsion-Solvent Evaporation Method
Spray Drying
Spray drying is a rapid and efficient method for preparing microspheres, particularly suitable for heat-stable drugs. In this technique, a solution or suspension of the drug and polymer is atomized into fine droplets using a spray dryer [26]. The droplets are then dried instantly in a hot air chamber, resulting in the formation of solid microspheres. Parameters such as feed rate, atomization pressure, and inlet/outlet temperature are critical to achieving uniform particle size and drug encapsulation. While this method is advantageous for its speed and ability to handle a wide range of materials, it may not be suitable for thermolabile drugs due to exposure to high temperatures [27].
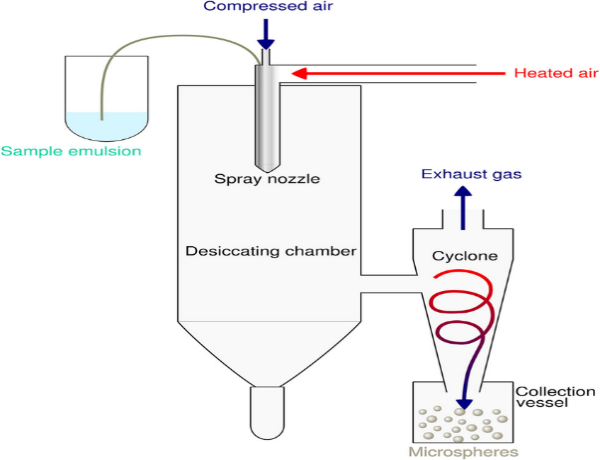
Figure 3: Spray Drying
Coacervation Phase Separation
This sophisticated method relies on inducing phase separation in a polymer solution to form a polymer-rich phase (coacervate) that encapsulates the drug. Typically, a nonsolvent or a salt is added to the polymer-drug solution to trigger phase separation. The coacervate droplets containing the drug are solidified by crosslinking or cooling, resulting in microsphere formation. This method allows for high encapsulation efficiency and precise control over particle characteristics, but it requires meticulous optimization of parameters such as temperature, solvent-nonsolvent ratio and stirring speed. Despite its advantages, coacervation is often complex and time-consuming [28].
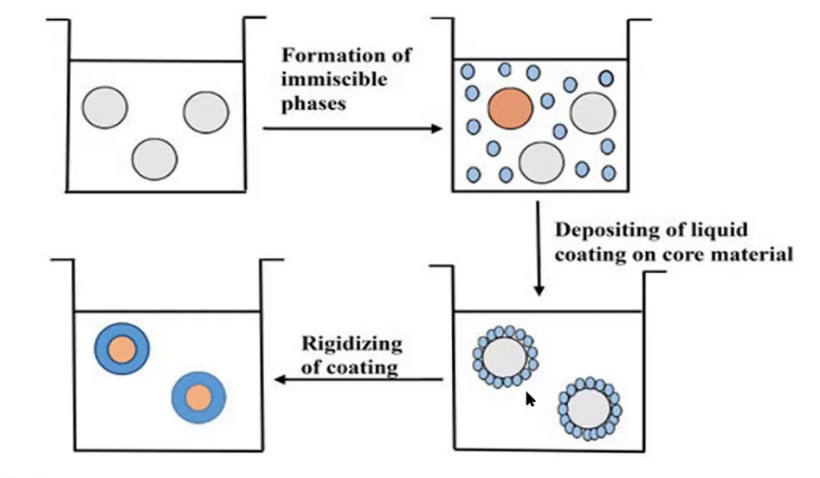
Figure 4: Coacervation Phase Separation
Ionic Gelation
Ionic gelation is a simple and environmentally friendly technique primarily used for natural polymers like alginate. The method involves dissolving the polymer in water and adding the drug to form a homogenous mixture. The mixture is then dropped into a crosslinking agent, such as calcium chloride solution, where the polymer undergoes ionic crosslinking to form solid microspheres. Parameters like crosslinking agent concentration, reaction time, and droplet size play a significant role in determining the properties of the microspheres [29]. This method is advantageous due to its solvent-free nature and biocompatibility but is limited to ionic polymers.
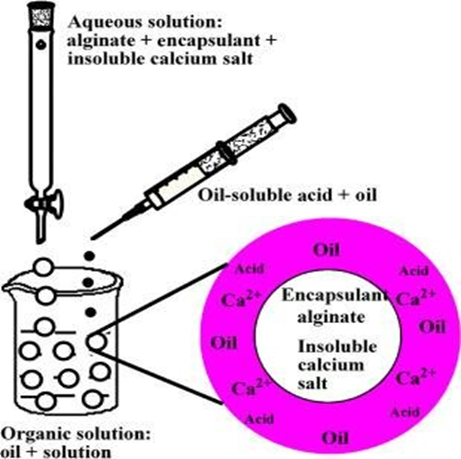
Figure 5: Ionic Gelation
Solvent Diffusion Method
In the solvent diffusion method, a partially miscible solvent containing the drug and polymer is introduced into a nonsolvent phase under continuous stirring. The solvent diffuses into the nonsolvent, leading to the precipitation of the polymer around the drug, forming microspheres. Key factors such as the solvent-nonsolvent ratio, diffusion rate, and stirring speed are critical for achieving uniform particle size and encapsulation efficiency. This method is highly efficient and results in microspheres with desirable characteristics, although precise control of solvent systems is necessary to ensure reproducibility [30].
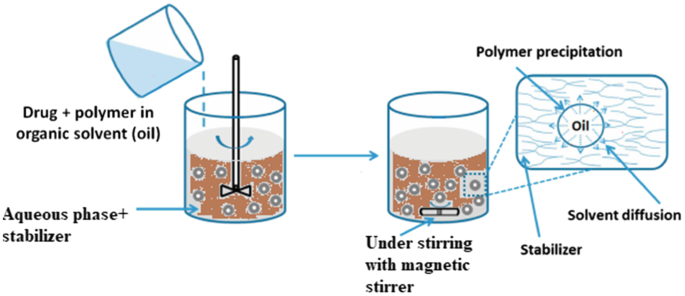
Figure 6: Solvent Diffusion Method
Evaluation Techniques
Characteristics
Characterizing these microparticulate carriers is a crucial phenomenon that aids in the creation of an appropriate and durable carrier for the transport of proteins, drugs, or antigens. The microstructures of each microsphere vary. The carrier's stability and release are determined by these microstructures [31].
Particle size and shape
Scanning electron microscopy (SEM), confocal fluorescence microscopy and conventional light microscopy (LM) are the most used methods for imaging microspheres. The form and external structure of microspheres can be ascertained using these methods. Conventional LM: For double-walled microspheres, conventional LM offers control over coating settings. Before and after coating, the architecture of the microsphere may be seen, and the change can be quantified under a microscope. Compared to LM, SEM offers superior resolution.
Scanning electron microscopy (SEM): SEM enables the examination of microsphere surfaces and, following particle cross-section, double-walled systems [32]. Confocal fluorescence microscopy: This technique is used to describe the structure of microspheres with numerous walls. Other than experimental techniques, laser light scattering and multi-size coulter counter can be employed to characterize the microspheres' size, shape, and morphology [33].
Angle of contact
The hydrophobicity and hydrophilicity of microspheres' wetting properties are determined by the angle of contact. The presence of an absorbed component affects this thermodynamic feature, which is specific to a solid substance. At the solid-air-water interface, the angle of contact is measured. A droplet is positioned in a circular cell above the objective of an inverted microscope to quantify the growing and decreasing angle of contact. Within a minute of the microspheres being deposited, the contact angle is measured at 200°C [33,34].
Fourier transform infrared (FT-IR) spectroscopy
The surface of the microspheres is ascertained by computing the alternating total reflectance (ATR), whereas FT-IR assesses the breakdown of the carrier system's polymeric matrix. In order to get IR spectra, primarily of surface material, an infrared light beam is directed through the ATR crystal in a way that causes it to reflect through the sample numerous times. The surface composition of the sample microspheres can be determined using ATR-FTIR in combination [35].
Density determination
The density of microspheres is measured using a multi-volume pycnometer. A precisely weighted sample of microspheres is prepared in a cup, which is subsequently put inside a multi-volume pycnometer. Helium gas is injected into the chamber and given time to expand while maintaining a steady pressure. The pressure inside the chamber drops as the helium gas expands, and two successive readings of the pressure drop at various starting pressures are recorded. The volume and density of the sample microsphere are calculated from these two pressure measurements [34].
Electron spectroscopy for chemical analysis (ESCA)
The microspheres' surface chemistry is determined by ESCA. By using electron spectroscopy to create spectra, ESCA also ascertains the atomic makeup of the microsphere's surface and the superficial degradation of biodegradable microspheres. Another name for it is X-ray photoelectron spectroscopy [34].
Surface carboxylic acid residue
The measurement of surface carboxylic acid residue is done using radioactive glycine. C14-glycine ethyl ester hydrochloride reacts chemically with the sample microspheres to create radioactive glycine conjugates. Ethyl-3 (3-dimethyl aminopropyl) carbodiimide (EDAC), a water-soluble condensing agent, is used to connect the glycine residue that is produced. The conjugate's radioactivity is then assessed using a liquid scintillation counter method. As a result, the carboxylic acid residue can be compared to the standard and conclusions made appropriately. Any derivatized kind of microsphere, whether hydrophobic or hydrophilic, can be measured using free carboxylic acid residue [35].
Isoelectric point
A device called micro electrophoresis, which evaluates the electrophoretic mobility of microspheres, is used to calculate the isoelectric point. The time it takes for particles to move across a distance of 1 mm is used to compute the mean velocity for each pH value between 3 and 10. With this information, the particle's electrical mobility can be ascertained. These three factors, such as the microspheres' ion-absorbing characteristics, ionizable behavior, or surface-contained charge, can all affect electrophoretic mobility [36].
Surface amino acid residue
The surface-associated amino acid residue is identified using the radioactive c14-acetic acid conjugate. Using a liquid scintillation counter, the carboxylic acid residue is first identified in order to indirectly determine the amino acid residue. The amino group and the carboxylic acid residue of c14-acetic acid are condensed using EDAC. By detecting the radioactivity of the c14 glycine ethyl ester hydrochloride with acetic acid or the glycine conjugates, the indirect estimation approach is used to identify the free amino or free carboxylic acid residues [36].
Capture efficiency
Allowing cleaned microspheres to come into contact with the lysate determines the microspheres' capture efficiency. The active ingredients in the formulation are then determined by testing the lysate in accordance with the guidelines provided in the specific monograph. The following formula is used to determine the percentage encapsulation efficiency [37]:
% Entrapment=Actual content/Theoretical content × 100
In vivo methods
Permeability on intact mucosa can be studied using in vivo techniques. These methods take use of the organism's biological reaction either locally or systemically. The systemic pharmacological effects that medications cause after being consumed or absorbed into the oral mucosa were used to estimate mucosal layer permeability in some of the earliest and most basic investigations. To research drug permeability, however, the most popular approaches nowadays are corneal perfusion chambers, buccal absorption studies and animal models [38].
Animal models
Animal models are used to screen a number of compounds in order to evaluate a range of formulations or look into the mechanisms and utility of permeation enhancers. There are a number of known animal models, including dogs, rats, rabbits, cats, hamsters, pigs, and sheep. The process entails giving the animal anesthesia and then administering the dose form that has to be studied. To stop absorption channels other than an oral mucosal layer, the rat esophagus is ligated. Blood is drawn at various times and analyzed to determine the absorption rate [39].
Buccal absorption test
It is renowned for its ease of use and dependability in determining the degree of medication loss in the oral cavity using both single- and multi-component drug mixes. This test method determines the solution's structure, contact time, pH and initial drug concentration when the drug is in the oral cavity [39].
Corneal perfusion chambers
The corneal perfusion chamber method is regarded as being extremely helpful in the creation and evaluation of ophthalmic medications. The goal of this research is to create and evaluate a modified perfusion chamber that can be used to apply medications that have been isolated to corneoscleral preparations topically and that enables ongoing endothelial cell function monitoring. This technique is appropriate for topical medication delivery since it uses a perfusion chamber made of stainless steel and polycarbonate to clamp corneas in a horizontal plane. During this perfusion, ultrasonic pachymetry and specular microscopy were used to evaluate the endothelial cell activity. The amount of fluorescein penetration was used to measure the function of the epithelial barrier. By evaluating the penetration of a big protein, leakage was investigated. Following perfusion, tissue architecture was analysed using traditional histology [40].
In vitro methods
Beaker method
This approach involves using an overhead stirrer to evenly mix the dosage form until it adheres to the bottom of the beaker containing the medium. The stirrer speed is between 60 and 300 rpm, and the amount of medium used is between 50 and 500 ml. The amount of drug dissolved in the media is measured by periodically withdrawing a sample [41].
Interface diffusion system
Dearden and Tomlinson created the interface diffusion system approach. There are four sections in it. The oral cavity, represented by Compartment A, has a drug concentration in a buffer that is suitable. The buccal membrane is represented by compartment B, which contains 1-octanol, while bodily fluids are represented by compartment C, which contains 0.2 M HCl. 1-octanol is also present in compartment D, which stands for protein binding. Prior to usage, 1-octanol and the aqueous phase were saturated with one another. Using a syringe, samples were taken out and put back in compartment A. As a result, samples from each of the four human bodily cavities are analyzed to detect the medication that has dissolved in each one [42].
Modified keshary chien cell
In the lab, a specific device was created. It was made up of a Keshary Chien cell with a dissolving medium of 50 milliliters of distilled water at 370 degrees Celsius. The majority of transmembrane drug delivery systems are stored in glass tubes with a 10# sieve at the bottom. The sieve is then reciprocated in the dissolution fluid at a rate of 30 strokes per minute, which determines the drug delivery system's rate of dissolution [43].
Dissolution apparatus
In vitro drug release profiles are studied using standard USP or BP dissolving apparatus, which uses both rotating parts (paddle 41, 42, 43, and basket 44, 45). The study's dissolution medium ranges from 100 to 500 milliliters, and its rotational speed ranges from 50 to 100 revolutions per minute [44].
Applications
Controlled and Sustained Drug Release
Microspheres are designed to release drugs at a controlled rate, prolonging the therapeutic effect and reducing dosing frequency. They prevent peak-and-trough plasma concentration fluctuations, thereby improving efficacy and patient compliance.
- Mechanism: Encapsulation of the drug in a polymer matrix (e.g., PLGA, ethyl cellulose) that degrades or allows diffusion over time.
- Examples:
- Propranolol hydrochloride-loaded microspheres for sustained hypertension management.
- Verapamil microspheres for extended cardiovascular therapy.
Targeted Drug Delivery
Microspheres enable site-specific drug delivery, minimizing systemic side effects and enhancing therapeutic efficacy. Techniques include ligand conjugation (active targeting) or magnetically controlled systems.
- Applications:
- Cancer Treatment: Magnetic microspheres loaded with doxorubicin for targeted chemotherapy.
- Hepatic Delivery: Liver-targeted delivery using galactose-functionalized microspheres.
Pulmonary Drug Delivery
Microspheres can deliver drugs directly to the lungs via inhalation. They are suitable for treating respiratory diseases due to their aerodynamic properties (1-5 µm particle size).
- Applications:
- Sustained delivery of corticosteroids for asthma and COPD.
- Tobramycin-loaded microspheres for cystic fibrosis-associated infections.
Gastroretentive Drug Delivery
Floating microspheres increase gastric residence time, improving the bioavailability of drugs with a narrow absorption window in the stomach or upper small intestine.
- Applications:
- Floating microspheres of ciprofloxacin for eradication of Helicobacter pylori.
- Ranitidine-loaded microspheres for sustained gastric ulcer treatment.
Ocular Drug Delivery
Microspheres improve ocular bioavailability by enhancing retention in the conjunctival sac or within the eye, ensuring prolonged therapeutic effects.
- Applications:
- Microspheres of pilocarpine for glaucoma management.
- Brimonidine-loaded microspheres for sustained intraocular pressure reduction.
Vaccines and Immunotherapy
Microspheres serve as antigen carriers, enhancing immune responses by sustained antigen release or by acting as adjuvants.
- Examples:
- PLGA microspheres encapsulating hepatitis B surface antigens.
- Tuberculosis vaccine formulations using biodegradable microspheres.
Cancer Therapy
Microspheres offer localized chemotherapy delivery to minimize damage to healthy tissues and reduce systemic toxicity.
- Examples:
- Paclitaxel-loaded microspheres for localized breast cancer treatment.
- Radioactive microspheres for hepatic carcinoma therapy (e.g., Yttrium-90).
Topical and Transdermal Drug Delivery
Microspheres enhance drug penetration through the skin or provide a reservoir for sustained release in transdermal applications.
- Applications:
- Microspheres containing diclofenac for topical pain relief.
- Transdermal delivery of fentanyl microspheres for chronic pain management.
Bone Regeneration and Orthopedics
Microspheres act as drug reservoirs or scaffolds in orthopedic applications, such as delivering antibiotics or growth factors for bone regeneration.
- Examples:
- Vancomycin-loaded microspheres for osteomyelitis.
- Growth factor-loaded microspheres in hydrogel scaffolds for bone repair.
Peptides and Protein Delivery
Microspheres protect sensitive peptides and proteins from enzymatic degradation and ensure controlled release.
- Examples:
- Insulin-loaded microspheres for non-invasive (oral or nasal) diabetes treatment.
- Bovine serum albumin (BSA) encapsulated microspheres for protein delivery studies.
Gene Therapy
Microspheres enable safe and effective delivery of genetic material (e.g., DNA, siRNA). They protect genetic payloads from degradation and ensure targeted delivery to cells.
- Examples:
- DNA-loaded microspheres for genetic disease correction.
- Microspheres delivering siRNA for silencing oncogenes in cancer therapy.
Oral Drug Delivery
Microspheres are widely used for sustained drug release in the gastrointestinal tract. They improve the bioavailability of poorly soluble drugs and reduce the frequency of dosing.
- Examples:
- Diclofenac sodium-loaded microspheres for chronic pain management.
- Clarithromycin microspheres for treating H. pylori infections.
Hormone Delivery
Microspheres provide a platform for sustained hormone delivery, ensuring consistent therapeutic effects for weeks or months.
- Examples:
- Leuprolide acetate microspheres for prostate cancer and endometriosis.
- Norethindrone microspheres for long-term contraception.
Antibiotic Delivery
Microspheres enable localized and controlled antibiotic release, improving efficacy and reducing the risk of antibiotic resistance.
- Examples:
- Gentamicin-loaded microspheres for wound infections.
- Ciprofloxacin microspheres for bacterial biofilm eradication.
Cardiovascular Applications
Microspheres are used in targeted delivery or sustained-release formulations for cardiovascular drugs, reducing side effects and improving therapeutic outcomes.
- Examples:
- Nifedipine-loaded microspheres for hypertension.
- Propranolol microspheres for beta-blocker therapy.
Cosmetic and Dermatological Applications
Microspheres are increasingly used in cosmetics for sustained release of active ingredients, improving product efficacy and user experience.
- Applications:
- Vitamin C microspheres in anti-aging creams.
- Retinol microspheres for acne treatment [45-48].
Figure 7: Applications of Microspheres
Future perspective
The future of microspheres as multiarticulate drug delivery systems holds great promise in personalized medicine, combination therapy, and targeted drug delivery. Advances in biodegradable and biocompatible polymers, along with the development of long-acting and intelligent drug release systems, will further enhance their therapeutic potential. Integration of smart microspheres that respond to environmental triggers and the clinical translation of these systems through scalable manufacturing and regulatory approvals are key areas of focus. As these innovations evolve, microspheres will offer more precise, effective, and patient-specific treatments, revolutionizing drug delivery for chronic and complex diseases.
CONCLUSION
The formulation and optimization of microspheres as a multiarticulate drug delivery system present a revolutionary advancement in the field of pharmaceutics. These systems offer numerous advantages, including controlled and sustained drug release, reduced side effects and targeted therapy. The ongoing research into new materials, advanced manufacturing techniques, and innovative design approaches holds great promise for addressing current challenges in drug delivery. Key considerations for the future include the need for improved biocompatibility, biodegradability, and cost-effectiveness of microsphere systems. Additionally, the integration of personalized medicine, combination therapies, and targeted delivery will be instrumental in maximizing the therapeutic outcomes of these formulations. Overall, the continued development of microsphere-based drug delivery systems could significantly improve the management of chronic diseases, enhance patient compliance, and offer more effective treatments with minimal adverse effects. As these systems progress from the laboratory to clinical settings, they will undoubtedly play a critical role in shaping the future of pharmaceutical therapies
REFERENCE
- Kakar S, Jain A. Magnetic microspheres: An Overview. Asian Pac J Health Sci 2019;6:81-9.
- Sharma M, Dev SK, Kumar M, Shukla AK. Microspheres as a suitable drug carrier in sustained release drug delivery: An overview. Asian J Pharm Pharmacol 2018;4:102-8.
- Nidhi P, Anamika C, Twinkle S, Mehul S, Hitesh J, Umesh U. Controlled drug delivery system: A review. Indo Am J Pharm Sci 2016;3:227-33.
- Prasad BS, Gupta VR, Devanna N, Jayasurya K. Microspheres as drug delivery system-a review. J Glob Trends Pharm Sci 2014;5:1961-72.
- Virmani T, Gupta J. Pharmaceutical application of microspheres: An approach for the treatment of various diseases. Int J Pharm Sci Res 2017;8:3252-60.
- Lengyel M, Kállai-Szabó N, Antal V, Laki AJ, Antal I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci Pharm 2019;87:20.
- Farraj NF, Johansen BR, Davis SS, Illum L. Nasal administration of insulin using bioadhesive microspheres as a delivery system. J Control Release 1990;13:253-61.
- Genta I, Conti B, Perugini P, Pavanetto F, Spadaro A, Puglisi G. Bioadhesive microspheres for ophthalmic administration of acyclovir. J Pharm Pharmacol 1997;49:737-42.
- Chandna A, Batra D, Kakar S, Singh R. A review on target drug delivery: Magnetic microspheres. J Acute Dis 2013;2:189-95.
- Zhang J, Zhang S, Wang Y, Zeng J. Composite magnetic microspheres: Preparation and characterization. J Magn Magn Mater 2007;309:197-201.
- Sangale SB, Barhate SD, Jain BV, Potdar M. Formulation and evaluation of floating felodipine microsphere. Int J Pharm Res Dev 2011;3:163-70.
- Srivastava AK, Ridhurkar DN, Wadhwa S. Floating microspheres of cimetidine: Formulation, characterization and in vitro evaluation. Acta Pharm 2005;55:277-85.
- De Cuyper M, Bulte JW, editors. Urs HÄfeli. In: Radioactive Microspheres for Medical Applications. Physics and Chemistry Basis of Biotechnology. Vol. 7. Springer: Dordrecht; 2001. p. 213-48.
- El-Helw AM, Al-Hazimi AM, Youssef RM. Preparation of sustained release phenobarbitone microspheres using natural and synthetic polymers. Med Sci 2008;15:39-51.
- Cai Y, Chen Y, Hong X, Liu Z, Yuan W. Porous microsphere and its applications. Int J Nanomed 2013;8:1111.
- Zhang CZ, Niu J, Chong YS, Huang YF, Chu Y, Xie SY, et al. Porous microspheres as promising vehicles for the topical delivery of poorly soluble asiaticoside accelerate wound healing and inhibit scar formation in vitro and in vivo. Eur J Pharm Biopharm 2016;109:1-3.
- Budov VV. Hollow glass microspheres. Use, properties, and technology. Glass Ceram 1994;51:230-5.
- Li S, Nguyen L, Xiong H, Wang M, Hu TC, She JX, et al. Porouswall hollow glass microspheres as novel potential nanocarriers for biomedical applications. Nanomed Nanotechnol 2010;6:127-36.
- Ratnaparkhi M, Wattamwar M, Jadhav A, Chaudhari S. Mucoadhesive microsphere-review. Int J Drug Dev Res 2014;6:975-1344.
- Degim IT, Çelebi N. Controlled delivery of peptides and proteins. Curr Pharm Des 2007;13:99-117.
- Bansal H, Kaur S, Gupta A. Microsphere: Methods of prepration and applications; a comparative study. Int J Pharm Sci Rev Res 2011;10:69-78.
- Wu L, Wang M, Singh V, Li H, Guo Z, Gui S, et al. Three-dimensional distribution of surfactant in microspheres revealed by synchrotron radiation X-ray microcomputed tomography. Asian J Pharm Sci 2017;12:326-34.
- Avachat A, Bornare P, Dash R. Sustained release microspheres of ropinirole hydrochloride: Effect of process parameters. Acta Pharm 2011;61:363-76.
- Ranjha NM, Khan H, Naseem S. Encapsulation and characterization of controlled release flurbiprofen loaded microspheres using beeswax as an encapsulating agent. J Mater Sci Mater Med 2010;21:1621-30.
- Pachuau L, Mazumder B. A study on the effects of different surfactants on ethyl cellulose microspheres. Int J Pharm Tech Res 2009;1:966-71.
- Kim JC, Song ME, Lee EJ, Park SK, Rang MJ, Ahn HJ. Preparation of microspheres by an emulsification-complexation method. J Colloid Interface Sci 2002;248:1-4.
- De Rosa G, Iommelli R, La Rotonda MI, Miro A, Quaglia F. Influence of the co-encapsulation of different non-ionic surfactants on the properties of PLGA insulin-loaded microspheres. J Control Release 2000;69:283-95.
- Gaur PK, Mishra S, Bajpai M. Formulation and evaluation of controlledrelease of telmisartan microspheres: In vitro/in vivo study. J Food Drug Anal 2014;22:542-8.
- Dinarvand R, Moghadam SH, Sheikhi A, Atyabi F. Effect of surfactant HLB and different formulation variables on the properties of poly-D, L-lactide microspheres of naltrexone prepared by double emulsion technique. J Microencapsul 2005;22:139-51.
- Shiga K, Muramatsu N, Kondo T. Preparation of poly (D, L?lactide) and copoly (lactide?glycolide) microspheres of uniform size. J Pharm Pharmacol 1996;48:891-5.
- Wan LS, Heng PW, Chan LW. Surfactant effects on alginate microspheres. Int J Pharm 1994;103:267-75.
- Khare P, Jain SK. Influence of rheology of dispersion media in the preparation of polymeric microspheres through emulsification method. AAPS Pharm Sci Tech 2009;10:1295-300.
- Kumbar SG, Kulkarni AR, Aminabhavi TM. Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: Effect of crosslinking agent. J Microencapsul 2002;19:173-80.
- Gülsu A, Ayhan H, Ayhan F. Preparation and characterization of ketoprofen loaded albumin microspheres. Turk J Biochem 2012;37:120-8.
- Jayan SC, Sandeep AV, Rifash M, Mareema CM, Shamseera S. Design and in vitro evaluation of gelatin microspheres of salbutamol sulphate. Hygeia 2009;1:17-20.
- 36. Wei W, Wang LY, Yuan L, Wei Q, Yang XD, Su ZG, Ma GH. Preparation and application of novel microspheres possessing autofluorescent properties. Adv Func Mater 2007; 17:3153-8.
- Behera AL, Patil SV, Sahoo SK. Formulation and characteristics of 5 fluorouracil microspheres by solvent evaporation method. Int J Pharm Pharm Sci 2011;3:32-5.
- Kendre P, Chaudhari P. Formulation and evaluation of telmisartan microspheres by solvent evaporation technique. Indo Am J Pharm Res 2012;2:651-7.
- Uyen NT, Hamid ZA, Tram NX, Ahmad N. Fabrication of alginate microspheres for drug delivery: A review. Int J Biol Macromol 2020;153:1035-46.
- Uyen NT, Hamid ZA, Ahmad NB. Synthesis and characterization of curcumin loaded alginate microspheres for drug delivery. J Drug Deliv Sci Technol 2020;58:101796.
- Mua L, Fenga SS. Fabrication, characterization and in vitro release of paclitaxel (TaxolÒ) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J Control Release 2001;76:239-54.
- Zalloum NL, de Souza GA, Martins TD. Single-emulsion P (HB-HV) microsphere preparation tuned by copolymer molar mass and additive interaction. ACS Omega 2019;4:8122-35.
- Yanga YY, Chiab HH, Chunga TS. Effect of preparation temperature on the characteristics and release profiles of PLGA microspheres containing protein fabricated by double-emulsion solvent extraction/ evaporation method. J Control Release 2000;69:81-96.
- Bhattacharya S, Alam M, Dhungana K, Yadav S, Chaudhary KR, Chaturvedi KK, et al. Preparation and evaluation of diclofenac gelatin microspheres using coacervation technique. Int J Pharm Res Innov 2020;13:14-21.
- Bertoni S, Albertini B, Passerini N. Different BCS Class II druggelucire solid dispersions prepared by spray congealing: Evaluation of solid state properties and in vitro performances. Pharmaceutics 2020;12:548.
- Gurung BD, Kakar S. An overview on microspheres. Int J Health Clin Res 2020;3:11-24.
- Baimark Y, Srisuwan Y. Preparation of polysaccharide-based microspheres by a water-in-oil emulsion solvent diffusion method for drug carriers. Int J Polym Sci 2013;2013:1-6.
- Kim JU, Shahbaz HM, Lee H, Kim T, Yang K, Roh YH, et al. Optimization of phytic acid-crosslinked chitosan microspheres for oral insulin delivery using response surface methodology. Int J Pharm 2020;588:119736


 Prajval Birajdar* 1
Prajval Birajdar* 1







 10.5281/zenodo.14740261
10.5281/zenodo.14740261