Abstract
Liquisolid compacts (LSCs) are a fascinating drug delivery technology that offers numerous advantages, particularly for Biopharmaceutical Classification System (BCS) class 2 drugs. In essence, they convert liquid medications into free-flowing, compressible powder forms suitable for tablet production with improved drug release characteristics. Formulation of liquisolid compacts represents a valuable strategy in overcoming solubility challenges, ultimately contributing to the development of effective and patient-friendly drug delivery system. This paper reviews the key components and the formulation process involved in developing liquisolid compacts. The improved wettability of the drug and the increased surface area of the molecularly dispersed drug in the liquid environment contribute to enhanced solubility. Pre- and post-compression tests are performed to optimize liquisolid compacts. Solid-state characterization studies (FTIR, DSC, SEM, PXRD) ensure drug-excipient compatibility. Accelerated stability studies demonstrate the safety and stability of liquisolid formulations over time. Thus, exploring different techniques and excipients can boost the pharmaceutical industry in designing stable and efficacious liquisolid compacts.
Keywords
Liquisolid Compacts, Quality by Design, Central Composite Design
Introduction
Liqui Solid Compacts are stable and cohesive mixture encompassing liquid and solid components in a way that results in a single tablet. The incorporation of excipients, such as carriers, binders, and disintegrants, plays a crucial role in achieving a balanced and effective formulation. The key components in the formulation, including the liquid medication, powdered excipients, and coating materials, play crucial roles in achieving the desired properties of the liquisolid compact 1-3. The incorporation of DoE and QbD approach along with Central Composite Design can bring about a more definite formulation by consideration of multiple factors and variables affecting the formulation.
Figure 1: Classification of Liquisolid Compacts 7
Benefits of LSCs:
- Enhanced dissolution: By finely dispersing the drug within the liquisolid system, LSCs can significantly improve drug dissolution rate and bioavailability, especially for poorly-soluble drugs 4. By converting liquid drugs into dry, free-flowing powders, liquisolid systems increase the wetting properties and surface area of the drug, leading to enhanced dissolution rates
- Controlled release: By using specific excipients and formulating strategies, LSCs can also be designed for sustained or modified drug release 5. Liquisolid compacts offer the flexibility to tailor drug release profiles by using suitable formulation excipients. By incorporating hydrophobic carriers for sustained release or surface-active agents for improved wettability, liquisolid systems can achieve specific release characteristics based on the formulation requirements
- Improved stability: LSCs can offer better protection against drug degradation compared to pure liquids 6. The liquid medication in liquisolid compacts can be protected from degradation by moisture or air exposure. This can be especially important for drugs that are sensitive to these environmental factors
- Cost-effective: LSCs utilize simple excipients and standard manufacturing processes, making them a cost-effective drug delivery option. Manufacturing liquisolid compacts is generally more cost-effective compared to soft gelatin capsules. The production cost of liquisolid systems is lower, making them an economically viable option for enhancing drug dissolution and bioavailability 7.
Components:
Carrier Material: This forms the backbone of the liquisolid compact, providing structure and facilitating compression into tablets. It's typically a high-porosity, high-compression material with a large surface area. Common choices include:
Microcrystalline Cellulose (MCC): The most widely used carrier due to its excellent compressibility, good flowability, and inert nature.
Starch: Another popular option with good compressibility and can be chosen based on desired disintegration properties (e.g., pregelatinized starch for faster disintegration).
Lactose: A cost-effective option with good flowability, but may have limitations for moisture-sensitive drugs.
Amorphous Cellulose: Offers high surface area for drug adsorption and potentially faster drug release 8,10.
Coating Material: This component plays a crucial role in absorbing and retaining the liquid medication within the compact. It's usually a fine, highly adsorbent material with a large surface area. Common choices include:
Colloidal Silicon Dioxide (Colloidal Anhydrous Silica): The most widely used coating material due to its high surface area, excellent adsorption capacity, and good flowability. Different grades with varying pore sizes can be chosen for specific drug-liquid medication combinations.
Microcrystalline Cellulose: Can also be used as a coating material in some cases, particularly when combined with colloidal silicon dioxide for a synergistic effect 9,11.
Liquid Medication: This is the core component containing the drug. It can be:
Drug Solution: The drug is dissolved in a suitable non-volatile solvent like propylene glycol or polyethylene glycol.
Drug Suspension: For drugs with limited solubility, a fine drug suspension in a non-volatile solvent can be used.
Emulsion: In some cases, an oil-in-water emulsion may be employed, especially for lipophilic drugs 12.
Formulation: Formulation Process:
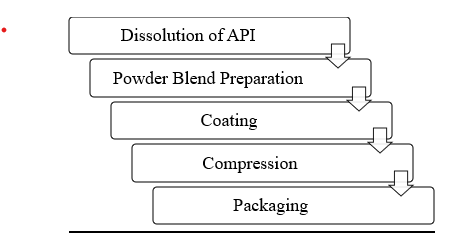
Figure 2:Stepwise methodology of Liquisolide Compact Preparation 19
Steps of Formulating LiquiSolid compacts
Dissolution of API:
Dissolve or disperse the poorly water-soluble drug in the selected liquid vehicle. The choice of solvent depends on the solubility of the drug 13.
Powder Blend Preparation:
Mix the liquid medication with the powdered excipients to form a homogenous, free-flowing powder blend. The liquid is absorbed by the carrier material. Mixing Technique involves
- Geometric Mixing:
Method: Geometric mixing involves blending the drug, liquid vehicle, and carrier material to create a homogenous mixture.
Advantages:
Simple and straightforward process.
Minimal equipment requirements.
Suitable for low-dose formulations.
Limitations:
Limited drug loading capacity due to carrier limitations.
May result in poor flow properties.
Application: Geometric mixing is commonly used for small-scale or lab-scale preparations.
- Wet Granulation:
Method: Wet granulation involves wetting the drug and carrier particles with a liquid binder, followed by granulation and drying.
Advantages:
Improved flowability and compressibility.
Enhanced drug loading.
Better content uniformity.
Limitations:
Requires additional processing steps (granulation and drying).
May involve use of solvents.
Application: Wet granulation is suitable for larger-scale production.
- Coevaporation:
Method: Coevaporation combines the drug and liquid vehicle by evaporating the solvent under controlled conditions.
Advantages:
High drug loading capacity.
Minimal impact on flow properties.
Efficient for lipophilic drugs.
Limitations:
Requires specialized equipment (rotary evaporator).
Solvent selection is critical.
Application: Coevaporation is preferred for high-dose formulations.14,15.
Comparative Analysis:
Drug Loading Capacity:
Wet granulation > Coevaporation > Geometric mixing
Flow Properties:
Geometric mixing < Wet>
Process Complexity:
Geometric mixing < Coevaporation>
Suitability for Scale-Up:
Coevaporation > Wet granulation > Geometric mixing
Table 1: Comparative Analysis of Geometric Mixing, Wet Granulation and Co-evaporation
|
Metric
|
Geometric Mixing
|
Wet Granulation
|
Coevaporation
|
|
Drug Dissolution
|
Studies needed to quantify 12, 37
|
May improve dissolution depending on binder used 14,39
|
Potentially high dissolution due to intimate mixing 15,44
|
|
Flowability
|
Generally good 11,38
|
May require additional processing for good flow 14,41
|
Excellent flowability due to spherical particles 15,43
|
|
Production Scalability
|
Simplest method, easily scalable 11,39
|
More complex than geometric mixing, scalability challenges 14,43
|
Requires specialized equipment, moderate scalability 15,45
|
|
Cost-Effectiveness
|
Simplest and potentially cheapest 12,40
|
More expensive than geometric mixing due to additional processing 13,42
|
Most expensive due to specialized equipment 15,44
|
- Coating:
Coat the surface of the powder particles with a hydrophilic material to prevent further absorption of liquid and improve the powder's flow properties.
- Compression:
Compress the powder blend into tablets or compacts using suitable compression equipment 16.
There is a certain variable that needs to be analysed which is Liquid Load Factor
Liquid Load Factor Calculation:
- Determine the maximum amount of liquid drug that can be absorbed by the carrier material while maintaining acceptable flowability and compressibility.
- This is called the "liquid load factor" or "R value" 17,18
Incorporation of CCD to optimize liquisolid compacts:
Identification of Factors:
Identify the factors that may influence the properties of liquisolid compacts. These can include the type and concentration of liquid vehicle, carrier material, coating agents, and other formulation parameters 20,21.
Selection of Levels:
Decide on the levels of each factor (low, medium, and high) based on the expected range for each variable. This is crucial for designing the experimental matrix.
Experimental Matrix:
Use the CCD to create a matrix of experimental runs. The matrix includes factorial points, axial points, and center points. The number of runs depends on the number of factors and levels chosen.
Preparation of Experiments:
Conduct the experiments according to the combinations specified in the experimental matrix. For each combination, prepare liquisolid compacts using the defined factors and levels 22,23.
Response Variables:
Define response variables related to the performance of liquisolid compacts, such as dissolution rate, drug release, compressibility, or other relevant properties.
Data Analysis:
Analyse the experimental data using statistical tools to determine the main effects and interaction effects of the factors on the response variables. This helps in identifying the optimal formulation.
Optimization:
Use the analysis results to identify the optimal formulation conditions that maximize the desired properties of liquisolid compacts.
Validation:
Validate the optimized formulation through additional experiments to ensure the reliability of the results.
Characterization parameters:
Content Uniformity:
Verify that the distribution of the active pharmaceutical ingredient (API) or other key components is consistent throughout the compact 24
Dissolution Rate:
Evaluate the dissolution profile of the liquisolid compact to ensure that the drug is released as intended. This is particularly crucial in pharmaceutical applications.
Physical Appearance and Organoleptic Properties:
Examine the overall appearance, color, and physical integrity of the compact. Ensure there are no signs of discoloration, caking, or other defects.
Hardness and Friability:
Measure the hardness of the compact, as it influences its mechanical strength. Additionally, assess friability to determine the tendency of the compact to break or crumble.
Particle Size and Surface Area:
Analyze the particle size distribution and surface area of the solid components in the formulation. This can impact the compact's dissolution and overall performance.
Flow Properties:
Evaluate the flow properties of the liquisolid powder blend. This is important for consistent and uniform tablet or compact formation during manufacturing.
Microscopy:
Examines drug distribution and particle morphology within the LSC matrix. Scanning electron microscopy (SEM) or transmission electron microscopy (TEM)
Moisture Content:
Determine the moisture content of the formulation to assess its stability and potential for degradation over time.
Hygroscopicity:
Evaluate the tendency of the compact to absorb moisture from the environment. Excessive hygroscopicity can affect the stability of the formulation.
In Vitro Drug Release:
Perform in vitro drug release studies to understand how the drug is released under simulated physiological conditions. This is crucial in pharmaceutical applications to predict the formulation's behaviour in the body.
Stability Studies:
Conduct stability studies under different storage conditions to assess the formulation's long-term stability and shelf life.
Compatibility Studies:
Investigate the compatibility of the liquisolid formulation with various excipients, especially in pharmaceuticals, to ensure no undesirable interactions occur.
X-ray Diffraction (XRD) and Differential Scanning Calorimetry (DSC):
Employ these techniques to investigate the physical state of the drug and excipients, ensuring there are no undesired changes in crystallinity.
Rheological Properties:
Assess the rheological properties of the liquid and powder components to understand their behavior during processing and manufacturing 25.
In vivo studies:
Assessment of pharmacokinetics and bioavailability in animal models.
Recent Advancements: Liquisolid compacts have been a promising area of research in drug delivery, particularly for poorly water-soluble medications. Here's a breakdown of recent advancements in this field:
- Novel Carrier Materials: Researchers are exploring new carrier materials beyond the traditional microcrystalline cellulose (MCC) and amorphous cellulose. Silica and its various forms are gaining traction due to their high adsorption capacity, leading to better powder flow in the final product 26-29.
- Beyond Dissolution Enhancement: The liquisolid technique isn't limited to just improving drug dissolution. Recent studies show promise in utilizing liquisolid compacts for sustained-release formulations. This allows for controlled drug release over a longer period, which can be beneficial for certain medications 30,31.
- Variations of the Technique: New variations of the liquisolid concept are emerging. Liquipellet is a technique derived from the liquisolid approach, involving extrusion and pelletization for better flowability and sustained release 32-34. Additionally, liquiground combines the advantages of co-grinding with liquisolid to enhance drug solubility
- Mesoporous silicas and clays have been used as efficient carrier and coating materials in liquisolid systems 35,36
QbD and DoE in Liquisolid Compact Formulation
QbD establishes a scientific framework for pharmaceutical development. It emphasizes understanding the relationship between product and process variables, and designing a process that consistently produces a quality product.
In the context of liquisolid compacts, QbD involves identifying the critical quality attributes (CQAs) of the product, such as drug release, dissolution rate, and tablet hardness. Then, it focuses on identifying the critical process parameters (CPPs) that can influence these CQAs. These CPPs typically include the ratio of drug to carrier material, the type and amount of coating material, and the processing conditions such as mixing time and compression force.
By using QbD, pharmaceutical scientists can develop a design space that defines the acceptable ranges for the CPPs to ensure consistent production of high-quality liquisolid compacts.
How DoE is used in liquisolid compacts
DoE is a collection of statistical techniques for designing experiments, analyzing data, and building models. It is a powerful tool that can be used to optimize the formulation of liquisolid compacts.
In liquisolid compact development, DoE can be used to:
- Identify the most important CPPs that affect the CQAs.
- Investigate the interactions between different CPPs.
- Develop a mathematical model that predicts the CQAs based on the CPPs.
- Optimize the formulation to achieve the desired product characteristics.
There are different types of DoE designs that can be used for liquisolid compact development, such as factorial designs, central composite designs, and Box-Behnken designs. The choice of design depends on the specific objectives of the study.
Benefits of using QbD and DoE in liquisolid compacts
- Reduced development time and cost: By using DoE, researchers can identify the most important factors affecting the product quality and optimize the formulation more efficiently.
- Improved product quality: QbD ensures a systematic approach to development, leading to a consistent and high-quality product.
- Regulatory compliance: QbD is becoming increasingly recognized by regulatory agencies as a valuable approach for pharmaceutical development.
CONCLUSION:
In nutshell we can conclude that liquisolid compacts can bring a paradigm shift in pharmaceutical industry as it has enhanced dissolution and bioavailability, improved stability, controlled release potential. The field continues to evolve, offering exciting possibilities for further enhancing the performance and applications of this innovative technology. The success of liquisolid compacts is evidenced by the comprehensive evaluation process, which encompasses various parameters. This innovative formulation strategy holds particular significance in the pharmaceutical industry, where the bioavailability of poorly water-soluble drugs often poses challenges. By improving drug solubility and dissolution, liquisolid compacts have the potential to enhance therapeutic outcomes and patient compliance. During compression, liquid drug may be squeezed out of a liquisolid tablet, resulting in unsatisfactory hardness. Exploring novel excipients or processing techniques to improve tablet hardness while maintaining drug content. Liquisolid systems require high solubility of the drug in non-volatile liquid vehicles.Developing strategies to overcome solubility limitations, such as using co-solvents or exploring alternative liquid carriers. Liquisolid powder should possess good flowability and compaction properties for large-scale production of capsules or tablets. Optimizing the balance between liquid content, flowability, and compaction properties for industrial-scale manufacturing.Limited invivo studies,Compatiblity and stability are some of the current challenges faced by the liquisolids.However further exploration of techniques and excipients can help in optimizing the dosage form.
REFERENCE
-
-
-
- Jaydip B, Dhaval M, Soniwala MM, Chavda J. Formulation and optimization of liquisolid compact for enhancing dissolution properties of efavirenz by using DoE approach. Saudi Pharmaceutical Journal. 2020 Jun 1;28(6):737-45.
- Naureen F, Shah Y, Shah SI, Abbas M, Rehman IU, Muhammad S, Goh KW, Khuda F, Khan A, Chan SY, Mushtaq M. Formulation development of mirtazapine liquisolid compacts: Optimization using central composite design. Molecules. 2022 Jun 22;27(13):4005.
- Jhaveri M, Nair AB, Shah J, Jacob S, Patel V, Mehta T. Improvement of oral bioavailability of carvedilol by liquisolid compact: optimization and pharmacokinetic study. Drug delivery and translational research. 2020 Aug;10:975-85.
- Ali B, Khan A, Alyami HS, Ullah M, Wahab A, Badshah M, Naz A. Evaluation of the effect of carrier material on modification of release characteristics of poor water soluble drug from liquisolid compacts. Plos one. 2021 Aug 2;16(8):e0249075.
- Dholakiya A, Dudhat K, Patel J, Mori D. An integrated QbD based approach of SMEDDS and liquisolid compacts to simultaneously improve the solubility and processability of hydrochlorthiazide. Journal of Drug Delivery Science and Technology. 2021 Feb 1;61:102162.
- Thakkar HP, Vasava D, Patel AA, Dhande RD. Formulation and evaluation of liquisolid compacts of itraconazole to enhance its oral bioavailability. Therapeutic Delivery. 2020 Feb;11(2):83-96.
- Mahammed N, Yadav H, Thulluru A, Narayana N, Balaji A, Mounika S. Formulation and Evaluation of Clopidogrel Bisulphate Tablets by Liquisolid Compact Technique. Research Journal of Pharmacy and Technology. 2020;13(5):2427-34.
- ] Vrushali G. Raut, Bharatee P. Chaudhari, Vivekkumar K. Redasani. Influence of Newly Synthesized Superdisintegrant on Dissolution Rate Enhancement of Carbamazepine using Liquisolid Compact Technique. Asian Journal of Research in Pharmaceutical Sciences. 2022; 12(2):107-4
- Sapana Ahirrao, Bhagyashree D. Gangode, Sanjay Kshirsagar. Solubility Enhancement of Ritonavir by using Liquisolid Compact Technique. Asian J. Pharm. Tech. 2017; 7 (4):189-201 .
- Patil IS, Patil OA, Rane NU, Nitalikar MM. A Review on Liquisolid Technique: A Novel Approach. Research Journal of Pharmaceutical Dosage Forms and Technology. 2018;10(3):188-92.
- Rane BR, Gaikwad DS, Jain AS, Pingale PL, Gujarathi NA. Enhancement of pioglitazone hydrochloride solubility through liquisolid compact formulation using novel carrier neusilin Us2. Pharmacophore. 2022 Jun 28;13(3):64-71.
- Gorle AP, Chopade SS. Liquisolid Technology: Preparation, Characterization and Applications. Journal of Drug Delivery and Therapeutics 2020;10:295–307. https://doi.org/10.22270/jddt.v10i3-s.4067.
- Lam M, Ghafourian T, Nokhodchi A. Liqui-Pellet: the Emerging Next-Generation Oral Dosage Form Which Stems from Liquisolid Concept in Combination with Pelletization Technology. AAPS PharmSciTech 2019;20. https://doi.org/10.1208/s12249-019-1441-9.
- De Espíndola B, Beringhs AOR, Sonaglio D, Stulzer HK, Silva MAS, Ferraz HG, et al. Liquisolid pellets: A pharmaceutical technology strategy to improve the dissolution rate of ritonavir. Saudi Pharmaceutical Journal 2019;27:702–12.
- Bhattacharya S. Central composite design for response surface methodology and its application in pharmacy. InResponse surface methodology in engineering science 2021 Jan 28. IntechOpen.
- Naureen F, Shah Y, Shah SI, Abbas M, Rehman IU, Muhammad S, Goh KW, Khuda F, Khan A, Chan SY, Mushtaq M. Formulation development of mirtazapine liquisolid compacts: Optimization using central composite design. Molecules. 2022 Jun 22;27(13):4005.
- Bonthagarala B, Dasari V, Kotra V, Swain S, Beg S. Quality-by-Design based development and characterization of pioglitazone loaded liquisolid compact tablets with improved biopharmaceutical attributes. Journal of Drug Delivery Science and Technology. 2019 Jun 1;51:345-55.
- Patil A, Kauthankar B, Kavatagimath S, Masareddy R, Dandagi P. Central Composite Design for the Development and Evaluation of Liquisolid Compacts of Glyburide. Indian Journal of Pharmaceutical Education and Research. 2022 Apr 1;56(2):S235-44.
- Regu V, Subudhi BB, Swain RP, Bhattacharjee A, Karna N, Khan AS, Nanda N. Formulation Development And Evaluation Of Liquisolid Compacts For Ibuprofen Liquisolid Tablets. Journal of Pharmaceutical Negative Results. 2023 Jan 1:559-71.
- Subramanian S, Monisha V. Investigating the use of liquisolid compact technique for pioglitazone HCI. Research Journal of Pharmacy and Technology. 2022;15(3):1013-7.
- Saeedi M, Akbari J, Enayatifard R, Morteza-Semnani K, Mohammad S, Hashemi H, Babaei A, Mashhadi SA, Eghbali M. Liquisolid Tablet: An Effective Approach Towards Improvement Of Dissolution Rate Of Famotidine As Poorly Soluble Drugs. Int. J. Pharm. Sci. Res. 2021;12(2):803-12.
- Bhavya E, Dhere MD. Liquisolid compacts technique of poor water soluble drugs: an overview. Research Journal of Pharmacy and Technology. 2021;14(10):5569-72.
- PRAJAPATI B, RAO MS, BAROT MT. Development, Optimization and Evaluation of Liquisolid Compact Tablet of Aripiprazole by using Factorial Design. International Journal of Pharmaceutical Research (09752366). 2020 Jul 1;12(3).
- Meka VS, Dharmanlingam SR, Kolapalli VR. Formulation of gastroretentive floating drug delivery system using hydrophilic polymers and its in vitro characterization. Brazilian Journal of Pharmaceutical Sciences. 2014 Apr;50:431-9.
- Shukla T, Pandey SP, Patil UK. Formulation of Gastroretentive Floating Drug Delivery System of Indomethacin. Indian J. Pharm. Educ. Res. 2014 Apr 1;48(2):41-7.
- Patil IS, Patil OA, Rane NU, Nitalikar MM. A Review on Liquisolid Technique: A Novel Approach. Research Journal of Pharmaceutical Dosage Forms and Technology. 2018;10(3):188-92.
- Khan I, Arjariya P, Sharma C, Sahni S, Sharma G, Gupta V. Liquisolid Technology: A Novel Concept. Asian Journal of Pharmaceutical Research and Development. 2017 Jan 1:1-7.
- Eryani MC, Hendradi E, Siswandono. Variation concentration effect of propyleneglycol, glycerin, and polyethyleneglycol 400 to physical properties and dissolution rate of loratadine liquisolid tablet. Journal of Basic and Clinical Physiology and Pharmacology. 2021 Jun 25;32(4):583-7.
- Deshmukh AS, Tiwari KJ, Mahajan VR. Solubility enhancement techniques for poorly water-soluble drugs. International Journal of Pharmaceutical Sciences and Nanotechnology (IJPSN). 2017 Jun 1:3701-8.
- Marathe BK, Gaurav GP, Dhangar V, Chatap VK. Preparation and Characterization of Pitavastatin Calcium Loaded Biodegradable Porous Starch as Carrier Platform for Drug Delivery. International Journal of Pharmaceutical Sciences and Nanotechnology (IJPSN). 2023 Nov 15;16(6):7049-56.
- Shid RL, Dhole SN, Kulkarni N, Shid SL. Formulation and evaluation of nanosuspension formulation for drug delivery of simvastatin. International Journal of Pharmaceutical Sciences and Nanotechnology (IJPSN). 2014 Nov 30;7(4):2650-65.
- Burra S, Yamsani M, Vobalaboina V. The Liquisolid technique: an overview. Brazilian journal of pharmaceutical sciences. 2011;47:475-82.
- Lu M, Xing H, Jiang J, Chen X, Yang T, Wang D, Ding P. Liquisolid technique and its applications in pharmaceutics. Asian journal of pharmaceutical sciences. 2017 Mar 1;12(2):115-23.
- Karmarkar A, Gonjari I, Hosman A, Dhabal P, Bhis S. Liquisolid tablets: a novel approach for drug delivery. International Journal of Health Research. 2009;2(1).
- Lam M, Ghafourian T, Nokhodchi A. Liquisolid system and liqui-mass system are not the same. AAPS PharmSciTech. 2020 Apr;21:1-3.
- Javadzadeh Y, Musaalrezaei L, Nokhodchi A. Liquisolid technique as a new approach to sustain propranolol hydrochloride release from tablet matrices. International journal of pharmaceutics. 2008 Oct 1;362(1-2):102-8.
- Nakaweh A, Al-Akayleh F, Al-Remawi M, Abdallah Q, Agha AS. Deep Eutectic System-Based Liquisolid Nanoparticles as Drug Delivery System of Curcumin for In-Vitro Colon Cancer Cells. Journal of Pharmaceutical Innovation. 2024 Apr;19(2):1-1.
- Shah S, Devani P, Dudhat K, Dudhrejiya A, Pashavan C, Mori D. Application of Liquisolid Pellets Technology for Improving Dissolution of Posaconazole: A DoE Based Process Optimization. Journal of Pharmaceutical Innovation. 2024 Jun;19(3):23.
- Abbas IK, Abddulhameed SN. Preparation and Characterization of Bilastine Solid Self-Nanoemulsion using Liquisolid Technique. Al-Rafidain Journal of Medical Sciences (ISSN 2789-3219). 2023 Jul 26;5:78-85.
- Bhagde S, Vaidya V, Mahajan M, Galgate A, Farande P, Jadhav R. Formulation and evaluation of liquisolid compacts of apixaban for anticoagulant activity. Latin American Journal of Pharmacy: A Life Science Journal. 2023 Jun 20;42(3):389-401.
- Tandale MS, Badave MP, Kolekar MS, Nagrale S, Pondkule MA, Babar V. The Liquid Technique: A Novel Approach to Improve the Solubility and Bioavailability of Insoluble Drugs.
- Regu V, Subudhi BB, Swain RP, Bhattacharjee A, Karna N, Khan AS, Nanda N. Formulation Development And Evaluation Of Liquisolid Compacts For Ibuprofen Liquisolid Tablets. Journal of Pharmaceutical Negative Results. 2023 Jan 1:559-71.
- Daravath B, Vemula SK. Liquisolid Technology: A Strategy for Dissolution Enhancement of BCS Class II Drugs.
- Teenadhayalan R, Vignesh S. Review On Liquisolid Compacts An Approach To Enhance The Solubility Of Bcs Class Ii Drugs. International Journal of Pharmaceutical Sciences. 2023 Nov 23;1(11):1-.
- Dhanaraju Md, Sundar Vd, Vadaga A, RAMYA N. Design, Formulation and optimization of liquisolid compact of Atazanavir by using DoE approach


 Priya Patil*
Priya Patil*


 10.5281/zenodo.14649685
10.5281/zenodo.14649685