Lecanemab, sold under the brand name Leqembi, is a monoclonal antibody medication used for the treatment of Alzheimer's disease.[2][4] Lecanemab is an amyloid beta-directed antibody.[2] It is given via intravenous infusion to patients with mild cognitive impairment or mild dementia.[2] In clinical trials, it demonstrated modest efficacy in reducing relative cognitive decline compared to placebo.[5] The most common side effects of lecanemab include headache, infusion-related reactions, and amyloid-related imaging abnormalities, a side effect known to occur with the class of antibodies targeting amyloid.[6]. Lecanemab was jointly developed by Eisai and Biogen. It was granted accelerated approval for medical use in the United States in January 2023[7] and fully approved by the FDA in July 2023[4][8].
APOE4; Alzheimer; clinical trial; immunotherapy; lecanemab
Lecanemab, also known by its brand name Leqembi, is a medication designed to treat Alzheimer's disease. It's a monoclonal antibody that targets amyloid beta, a substance that forms plaques in the brains of people with Alzheimer's. In clinical trials, lecanemab has shown modest efficacy in reducing cognitive decline in patients with early-stage Alzheimer's disease. The medication is administered via intravenous infusion every two weeks. However, lecanemab's use has been associated with some safety concerns, including amyloid-related imaging abnormalities (ARIA), which can cause swelling and bleeding in the brain. The risk of ARIA is higher in people with a certain genetic variation, known as ApoE4. Despite these concerns, the US FDA granted accelerated approval for lecanemab in January 2023, and later converted it to traditional approval in July 2023. However, the European Medicines Agency (EMA) recommended refusing marketing authorization for lecanemab in July 2024, citing concerns over its benefits and risks. Alzheimer's disease (AD) is a complex and debilitating neurodegenerative disorder affecting millions of people worldwide. Despite extensive research, the development of effective treatments for AD has been challenging. Recently, lecanemab, a humanized monoclonal antibody targeting amyloid beta, has emerged as a promising therapeutic candidate. This review aims to provide an overview of lecanemab's mechanism of action, clinical efficacy, and safety profile, as well as its potential role in the management of Alzheimer's disease.
Lecanemab is indicated for the treatment of Alzheimer's disease in people who have mild cognitive impairment or mild dementia, but not in people who already have moderate or severe dementia. [2][4][6]
Efficacy: In a phase III clinical trial of 1,795 patients aged 50 to 90 years old with early-stage Alzheimer’s disease, lecanemab slowed clinical decline by 27?ter 18 months of treatment compared with those who received a placebo [9] [10] The mean CDR-SOB score at baseline was approximately 3.2 among the study population, and the mean change from baseline after 18 months was +1.21 with lecanemab and +1.66 with placebo. (For the comparison, CDR-SOB score is 0 for the Normal level, 0.5–2.5 for Questionable impairment, 3.0–4.0 for Very mild dementia, 4.5–9.0 for Mild dementia, 9.5–15.5 for Moderate dementia, and 16.0–18.0 for Severe dementia.)[11] The authors concluded "Lecanemab reduced markers of amyloid in early Alzheimer’s disease and resulted in moderately less decline on measures of cognition and function than placebo at 18 months but was associated with adverse events."[10]
What is Alzheimer’s?
Definition and Overview:
Alzheimer's disease (AD) is a progressive, irreversible, and degenerative neurological disorder that affects memory, thinking, and behavior. It is the most common form of dementia, accounting for 60-80% of dementia cases.
Causes and Risk Factors:
The exact cause of Alzheimer's disease is still unknown, but research has identified several risk factors and potential causes:
1. Genetics: Family history and genetic mutations (e.g., APOE-e4) can increase the risk of developing AD.
2. Aging: The risk of AD increases with age, with most cases occurring after the age of 65.
3. Brain Plaques and Tangles: The accumulation of beta-amyloid plaques and tau protein tangles in the brain is a hallmark of AD.
4. Neuroinflammation: Chronic inflammation in the brain may contribute to the development and progression of AD.
5. Lifestyle Factors: Sedentary lifestyle, poor diet, and lack of social engagement may increase the risk of AD.
6. Medical Conditions: Certain medical conditions, such as diabetes, hypertension, and stroke, may increase the risk of AD.
Symptoms and Progression:
The symptoms of Alzheimer's disease can vary from person to person, but typically progress through several stages:
1. Early-Stage: Mild memory loss, difficulty learning new information, and mood changes.
2. Moderate-Stage: Noticeable cognitive decline, difficulty with communication, and increased memory loss.
3. Late-Stage: Severe cognitive decline, difficulty with daily activities, and increased dependence on caregivers.
4. End-Stage: Near-total cognitive decline, loss of motor skills, and increased risk of infections and other health complications.
Diagnosis and Treatment:
Diagnosing Alzheimer's disease can be challenging, but typically involves:
1. Medical History: Reviewing the patient's medical history and conducting a physical examination.
2. Cognitive and Neuropsychological Tests: Assessing cognitive function, memory, and thinking abilities.
3. Imaging Tests: Using MRI or CT scans to rule out other potential causes of symptoms.
4. Genetic Testing: Identifying genetic mutations that may increase the risk of AD.
While there is currently no cure for Alzheimer's disease, various treatments can help manage its symptoms:
1. Cholinesterase Inhibitors: Medications that increase the levels of acetylcholine in the brain, improving cognitive function.
2. Memantine: A medication that blocks the action of glutamate, a neurotransmitter that can be toxic to brain cells.
3. Combination Therapy: Using a combination of medications to manage symptoms.
4. Non-Pharmacological Interventions: Providing emotional support, managing behavioral symptoms, and promoting cognitive function through activities and exercises.
Prevention and Future Directions:
While there is no surefire way to prevent Alzheimer's disease, research suggests that:
1. Healthy Lifestyle: Maintaining a healthy diet, exercising regularly, and managing stress may reduce the risk of AD.
2. Cognitive Stimulation: Engaging in mentally stimulating activities, such as reading and puzzles, may help build cognitive reserve.
3. Social Engagement: Maintaining social connections and building strong relationships may support cognitive health.
Future directions for Alzheimer's research include:
1. Early Detection and Diagnosis: Developing biomarkers and imaging tests to detect AD at an early stage.
2. Personalized Medicine: Tailoring treatments to individual patients based on their genetic profiles and medical histories.
3. Novel Therapies: Exploring new therapeutic approaches, such as immunotherapy and gene therapy, to target the underlying causes of AD.
Pathophysiology of Alzheimer’s disease:
Amyloid Cascade Hypothesis:
The amyloid cascade hypothesis is the most widely accepted theory of Alzheimer's disease pathogenesis. It proposes that:
1. Amyloid Precursor Protein (APP): APP is a transmembrane protein that is cleaved by enzymes to produce beta-amyloid peptides.
2. Beta-Amyloid Aggregation: Beta-amyloid peptides aggregate to form insoluble fibrils, which deposit in the brain as senile plaques.
3. Tau Protein Hyperphosphorylation: The aggregation of beta-amyloid triggers the hyperphosphorylation of tau protein, leading to the formation of neurofibrillary tangles.
4. Neuroinflammation and Oxidative Stress: The deposition of beta-amyloid and tau protein triggers an inflammatory response, leading to the activation of microglia and the production of reactive oxygen species.
5. Neurodegeneration: The chronic inflammation and oxidative stress lead to the degeneration of neurons, resulting in cognitive decline and dementia.
Other Pathophysiological Mechanisms:
In addition to the amyloid cascade hypothesis, other mechanisms have been implicated in the pathogenesis of Alzheimer's disease:
1. Cholinergic Hypothesis: The degeneration of cholinergic neurons in the basal forebrain contributes to cognitive decline.
2. Glutamatergic Hypothesis: The dysregulation of glutamate neurotransmission contributes to excitotoxicity and neuronal damage.
3. Vascular Hypothesis: Cerebrovascular disease and reduced blood flow to the brain contribute to cognitive decline.
4. Mitochondrial Dysfunction: Mitochondrial dysfunction and reduced energy metabolism contribute to neuronal damage and degeneration.
5. Epigenetic Modifications: Epigenetic modifications, such as DNA methylation and histone modification, contribute to the regulation of gene expression and the development of Alzheimer's disease.
Key Players in Alzheimer's Disease Pathogenesis:
Several key players have been identified in the pathogenesis of Alzheimer's disease:
1. Beta-Amyloid: Beta-amyloid peptides are the main component of senile plaques and play a central role in the amyloid cascade hypothesis.
2. Tau Protein: Tau protein is a microtubule-associated protein that is hyperphosphorylated in Alzheimer's disease, leading to the formation of neurofibrillary tangles.
3. APOE: The APOE gene is a major genetic risk factor for Alzheimer's disease, with the APOE-e4 allele increasing the risk of developing the disease.
4. Presenilin: Presenilin is a protein that is involved in the processing of APP and the production of beta-amyloid peptides.
5. Microglia: Microglia are the resident immune cells of the brain and play a key role in the inflammatory response and the clearance of beta-amyloid peptides.
Mechanism of Action: Lecanemab
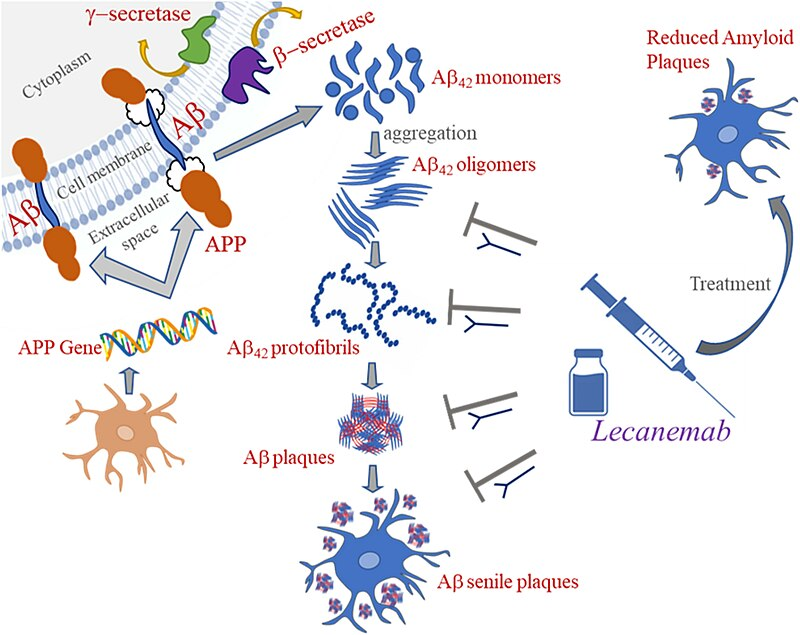
Figure a Mechanism of action of lecanemab
Lecanemab is a humanized IgG1 form of the murine antibody mAb158 that primarily targets soluble A? protofibrils while still being active against insoluble fibrils (Figure 1). A? protofibrils are large, soluble A? aggregates that cause neurotoxicity by interfering with the electrophysiological systems involved in memory function.2 Lecanemab has been demonstrated to decrease pathogenic A?, prevent A? deposition, and specifically diminish A? protofibrils in the brain and CSF of animal models of AD.5,6 The selective target of A? protofibrils separates lecanemab from other anti-amyloid mAbs. Lecanemab is the most efficient second-generation mAb for immunodepletion of A? protofibrils, particularly soluble protofibrils and oligomers. Lecanemab binds tiny protofibrils with 100 times the affinity of aducanumab and big protofibrils with 25 times the affinity, with lower binding affinity for monomers.7 In humans, lecanemab has been evaluated in several clinical trials. However, there is a need for a systematic review of the available evidence to evaluate the efficacy and safety of lecanemab in AD.
History:
In July 2022, the US Food and Drug Administration (FDA) accepted an application for accelerated approval for lecanemab. [15]
In September 2022, Biogen announced [15] [16] positive results from an ongoing phase III clinical trial. [17] [18]
In November 2022, it was announced that the drug was a success in clinical trials, and exceeded its goal in reaching primary endpoints. [19]


 Tejaswini Gurud *
Tejaswini Gurud *
 Madhura Jadhav
Madhura Jadhav


 10.5281/zenodo.14235128
10.5281/zenodo.14235128