Abstract
Drug discovery is the systematic procedure of identifying a chemical compound that possesses therapeutic properties for the purpose of healing and treating diseases. This process involves the identification of candidates, the synthesis, characterization, validation, optimization, screening, and testing to assess the therapeutic effectiveness. After a molecule has proven its significance in these investigations, it will initiate the process of developing medication. Preclinical development precedes clinical testing. The process of developing a new drug must advance through several stages in order to create a medication that is both safe and effective, and that meets all regulatory requirements. The essay highlights the lengthy, difficult, and costly nature of producing new medicines. It underscores the need to consider various biological targets for each authorized treatment. The validation of analytical method is essential for its development, whereby it is extensively tested for specificity, linearity, accuracy, precision, range, limit of detection, limit of quantitation, and robustness. Thus, the development and validation of analytical methods allows one to confirm that accurate and reliable measurement of the potency a pharmaceutical preparation can be performed. The present review highlights the process of drug development, its phases, and analytical methods, including chromatographic, spectroscopic, and electrochemical techniques, which have been applied in the analysis of pharmaceuticals.
Keywords
Drug development, therapeutic agent, biologics, small molecules, artificial intelligence
Introduction
Developing a new drug from an original idea to the launch of a finished product is a complex process which can take 12–15 years and cost in excess of $1 billion. The idea for a target can come from a variety of sources including academic and clinical research and from the commercial sector. It may take many years to build up a body of supporting evidence before selecting a target for a costly drug discovery program. Once a target has been chosen, the pharmaceutical industry and, more recently, some academic centers would have streamlined a number of early processes to identify molecules which possess suitable characteristics to make acceptable drugs. This review will look at key preclinical stages of the drug discovery process, beginning with initial target identification and validation. The primary focus of this review is on general approaches and considerations toward development of analytical methods for separation, identification, and quantification of active pharmaceutical compounds (APIs), which may be applied within various functions in the drug development continuum. The review also discusses the issues and parameters that must be considered in the validation of analytical methods.
DRUG DEVELOPMENT
Drug discovery and development is a knowledge-intensive process that implies the generation, management, and analysis of huge amounts of data from the initial target discovery research to the post-marketing pharmacovigilance studies, including central operations such as virtual screening and toxicological assessment.
DRUG DEVELOPMENT PROCESS FLOW CHART:
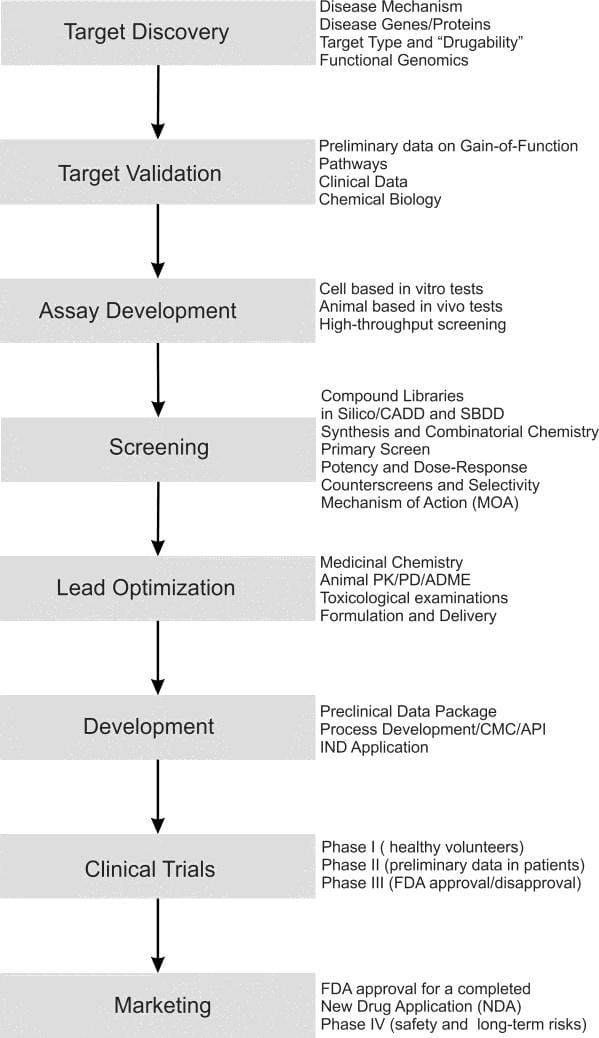
TYPES OF DOSAGE FORM

ROUTE OF DEUG ADMINISTRATION:
There are many different drug routes of administration. Some are commonly used, while others are rare. Drug administration can be:
oral, which is when a person swallows a drug intraocular, or into the eye intraotic, or into the ear nasal, or through the nose sublingual, or under the tongue buccal, between the gums and the mouth cheek inhaled through the respiratory system enteral, which is when a person receives the drug directly into their digestive tract rectal, or through the rectum vaginal, or through the vagina transdermal, or through the skin subcutaneous, or under the skin intramuscular, or via an injection into a muscle intravenous, or into a vein intra-arterial, or into an artery intraosseous, or into the bone marrow
Drug testing:
It is done primarily to screen people systematically or randomly for evidence of use of one or more substances with potential for abuse. Testing may be done in the following:
- Certain groups of people, commonly including students, athletes, and prisoners.
- People who are applying for or who already hold certain types of jobs (eg, pilots, commercial truck drivers).
- People who have been involved in motor vehicle or boating accidents or accidents at work.
- People who have attempted suicide by unclear means.
- People in a court-ordered treatment program or with terms of probation or parole requiring abstinence (to monitor adherence).
- People in a substance abuse treatment program (as a standard feature, to obtain objective evidence about substance abuse and thus optimize treatment).
- People required to participate in a drug testing program as part of custody or parental rights.
- Members of the military.
FORMULATED:
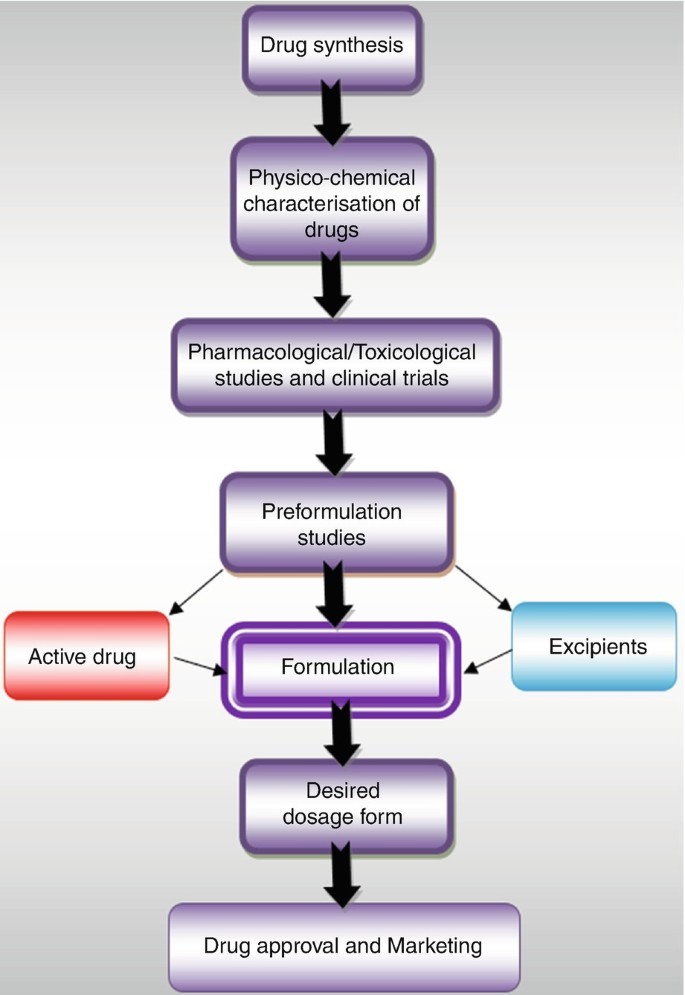
PRODUCE:
Extraction from plants:
Traditionally, drugs were extracted from medicinal plants.
Organic synthesis:
Drugs can also be produced through organic synthesis.
Drug discovery:
Modern drug discovery involves identifying screening hits, medicinal chemistry, and optimizing those hits to increase their efficacy, selectivity, and other properties.
Manufacturing:
Pharmaceutical manufacturers use a Site Master File (SMF) to document the production and control of their manufacturing operations.
The process of drug discovery is complex and involves many factors, including:
- Investors
- Industry
- Academia
- Patent laws
- Regulatory exclusivity
- Marketing
- The need to balance secrecy with communication.
REGULATED:
Drugs are regulated by government agencies to ensure their safety and efficacy. Regulatory bodies perform various functions, including: Licensing new medicines, Regulating clinical trials, Regulating herbal and homeopathic medicines, and Inspecting and maintaining standards of drug development and manufacture.
Some examples of regulatory bodies include:
Central Drugs Standard Control Organization (CDSCO): The regulatory body for drugs, pharmaceuticals, and medical devices in India. CDSCO is responsible for:
- Approving new drugs
- Conducting clinical trials
- Laying down standards for drugs
- Controlling the quality of imported drugs
Food & Drug Administration (FDA):
The regulatory body for drugs in the US
European Medicines Agency (EMA):
The regulatory body for drugs in Europe
Drug regulation is important because drugs can cause harm, such as: toxic impurities, unrecognized severe adverse reactions, adulterated drug products, and fake or counterfeit drugs.
SCHEDULE Y:
Schedule Y is a schedule in the Drugs and Cosmetics Act of India that provides guidelines for the conduct of clinical trials of new drugs in India. Schedule Y was first introduced in 1983 and has since been amended several times to align with the latest scientific and regulatory requirements.
GOOD MANUFACTURING PRACTICES (GMP):
Schedule M is a part of the Drugs and Cosmetics Act, 1945 that outlines Good Manufacturing Practices (GMP) for pharmaceutical products. GMP is a set of mandatory standards that ensure the quality of a product by controlling the materials, methods, machines, processes, personnel, and facility/environment. The Health Ministry recently revised Schedule M to align GMP recommendations with global standards, particularly those of the World Health Organization (WHO).
GOOD LABORATORY PRACTICE (GLP):
Good Laboratory Practice (GLP) is a quality system that ensures the safety and quality of non-clinical laboratory studies for pharmaceuticals and other products. Purpose GLP establishes that a product is safe for humans, animals, and the environment.
Scope
GLP applies to preclinical studies on chemicals, pharmaceuticals, veterinary medicines, pesticides, and biocides.
Process
GLP defines rules and criteria for planning, performing, monitoring, recording, reporting, and archiving studies.
Areas of focus
GLP monitors resources, characterization, rules, and results.
Examples of GLP practices
Wearing personal protective equipment (PPE), cleaning equipment regularly, labeling workspaces, and avoiding working alone.
APPLICATION OF NEW DRUG ADMINISTRATION:
A New Drug Application (NDA) is a request from a pharmaceutical manufacturer to the U.S. Food and Drug Administration (FDA) to market a new drug. The NDA is used to demonstrate that the drug is safe and effective for its intended use. The FDA uses the information in the NDA to determine:
- the drug is safe and If effective
- If the drug's benefits outweigh its risks
- If the proposed drug label is appropriate
- If the drug manufacturing standards are adequate
ADVANTAGES:
New drug development has many advantages, including:
Improved healthcare outcomes
drugs can treat or prevent diseases, improve the safety and efficacy of existing drugs, and advance personalized medicine.
Better patient care
New drugs can provide patients with up-to-date care and improve their quality of life.
Increased knowledge
New drug development can lead to a general increase in knowledge about healthcare, treatments, or drugs.
Fight against new and well-known diseases
New drugs can help fight against diseases like COVID-19 and cardiovascular disease, which kill millions of people each year.
Treat previously untreatable conditions
New drugs can treat conditions that were previously untreatable.
Optimized drug formulations
Optimized drug formulations can lead to enhanced efficacy, reduced side effects, and better patient compliance.
DISADVANTAGES:
Time-consuming
The process of developing a new drug can take years, or even a decade or more, to reach the market.
Expensive
The extensive testing and trials required to ensure a drug's safety and effectiveness can cost hundreds of millions of dollars.
Labor-intensive
The traditional discovery process requires a high level of expertise and is labor-intensive.
Animal models
The accuracy of animal models in predicting human responses to drugs is uncertain. This can lead to drug candidates being incorrectly identified as safe or unsafe for humans.
Heterogeneity of patient population
One-size-fits-all drugs are limited in success due to the heterogeneity of the patient population.
Lack of validated biomarkers
There is a lack of validated diagnostic and therapeutic biomarkers.
Complexity of data
The drug discovery process faces challenges due to the increasing complexity and array of data types.
Traditional organizational structures
Hierarchical and siloed departments in pharmaceutical companies can impede the flow of information and collaboration.
FUTURE PERSPECTIVE:
Technology
Pharmacists may need to adapt to new technologies, such as robots that dispense medications, 3D printers that create combination therapies, and algorithms that address clinical edits.
Patient care
Pharmacists may play a more meaningful role in patient care, including chronic condition management, specialty care, and primary care.
Digital health
Pharmacists may help patients find digital health products, set them up, and educate them on how to use them.
AI
AI technology may be used to generate human-like responses to natural language inputs, allowing patients to communicate with pharmacies in a conversational manner.
Collaboration
Pharmacists may collaborate with physicians to provide accessible patient care, especially for chronic conditions.
Burnout
Pharmacists may need to address burnout, which has been a problem for the profession in recent
REGULATORY GUIDELINES
Pharmaceutical regulations are a set of guidelines that ensure the safety and effectiveness of drugs, and help improve access to medically useful drugs. These regulations apply to every stage of drug development, from manufacturing to marketing.
Some examples of regulatory guidelines for pharmaceuticals include:
Current Good Manufacturing Practice (CGMP)
A critical guideline that requires drug manufacturers to follow strict standards for product quality. This includes keeping facilities clean, training employees properly, and documenting manufacturing processes.
International regulatory standards from the World Health Organization (WHO)
These standards cover topics such as stability, packaging, storage, and bioequivalence. The WHO also supports generic products and establishing bioequivalence standards between generic and originator medicines.
FDA 21 CFR Part 210
Also known as the Current Good Manufacturing Practice (cGMP) in Manufacturing, Processing, Packing, or Holding of Drugs, this regulation sets the standard for quality in the pharmaceutical
CONCLUSION
In this course you have had a brief introduction to how the drug-discovery process is conceived and initiated. You should by now have some appreciation of the intrinsic difficulties associated with developing a drug molecule from conception into a medicine suitable for clinical use and of how molecular modelling can be used in the drug discovery process. You should also by now have an elementary appreciation of the concept of pain and some understanding of the historical background to pain control. You should be aware that opioids, steroids and NSAIDs all work at the molecular level. Finally you should appreciate that anti-inflammation drug discovery requires a detailed understanding of the biochemical processes that constitute the inflammatory response.
REFERENCE
- Deore, AB, Dhumane JR, Wagh HV, Sonawane RB, The Stages of Drug Discovery and Development Process. Asian Journal of Pharmaceutical Research and Development. 2019; 7(6):62-67
- Shayne CG. Introduction: drug Discovery in the 21stCentury. Drug Discovery Handbook, Wiley Press, 2005; 1-10.
- Smith GC, OíDonnel JT. The Process of New Drug Discovery and Development, Eds., 2nd edition, Informa Healthcare, New York 2006.
- Moffat J, Vincent F, Lee J, Eder J, Prunotto M. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nature Reviews Drug Discovery, 2017; 16(8):531-543.
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. Journal of Health Economics, 2003; 151-185.
- Gashaw I, Ellinghaus P, Sommer A, Asadullah K. What makes a good drug target. Drug Discovery Today, 2012; 17:S24-S30.
- Lindsay MA. Target discovery. Nature Reviews Drug Discovery, 2003; 2:831–838.
- Terstappen G, Schlüpen, C, Raggiaschi R, Gaviraghi G. Target deconvolution strategies in drug discovery. Nature Reviews Drug Discovery, 2007; 6(11):891-903.
- Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nature Reviews Drug Discovery, 2006; 5:821- 834.
- Odilia Osakwe. Social Aspects of Drug Discovery, Development and Commercialization. Chapter 6 Preclinical In Vitro Studies: Development and Applicability. Elsevier. 2016.
- Henning SW, Beste G. Loss-of-function strategies in drug target validation. Current Drug Discovery Technology, 2002; 17–21.
- John GH, Martyn NB, Bristol-Myers S. High throughput screening for lead discovery. Burger?s Medicinal Chemistry and Drug Discovery, 6 the dition, Drug Discovery and Drug Development, Wiley Press, 2002; 2:37-70.
- Patidar AK, Selvam G, Jeyakandan M, Mobiya AK, Bagherwal A, Sanadya G, Mehta R. Lead Discovery and lead optimization: A useful strategy in molecular modification of lead compound in analog design. International journal of drug design and discovery. 2011; 2(2):458- 463.
- Huber W. A new strategy for improved secondary screening and lead optimization using high-resolution SPR characterization of compound–target interactions. J Mol. Recogn. 2005; 18:273–281.
- Lofas S. Optimizing the hit-to-lead process using SPR analysis. Assay of Drug Development Technologies, 2004; 2:407-416.


 Aditi Mudgal *
Aditi Mudgal *





 10.5281/zenodo.14249519
10.5281/zenodo.14249519