Abstract
The development of sustained release (SR) matrix tablets has garnered significant attention in modern pharmaceutical research owing to their ability to maintain prolonged therapeutic action, improve patient compliance, and enhance drug bioavailability. Among various approaches for achieving sustained release, the use of natural gums as matrix-forming agents has emerged as a promising strategy due to their biocompatibility, biodegradability, non-toxicity, and economic viability. This review focuses on the formulation, evaluation, and applications of SR matrix tablets, with a special emphasis on natural gums such as xanthan gum, guar gum, alginate, and karaya gum. The mechanisms of drug release from these hydrophilic matrices, including swelling, erosion, and diffusion, are discussed in detail. Key formulation parameters such as polymer concentration, drug solubility, and tablet hardness, which influence drug release kinetics, are analyzed. Furthermore, regulatory considerations, challenges in large-scale production, and potential future directions, such as the incorporation of nanomaterials or hybrid systems, are explored. By highlighting the critical role of natural gums in SR drug delivery, this review provides a comprehensive resource for researchers engaged in the development of advanced oral drug delivery systems.
Keywords
Sustained Release, Tablets, Delivery, Systems.
Introduction
In recent years, the demand for sustained release (SR) drug delivery systems has surged due to their potential to enhance therapeutic efficacy, reduce dosing frequency, and improve patient compliance. Unlike conventional dosage forms, which release their active pharmaceutical ingredients (APIs) immediately, sustained release systems are designed to provide a gradual release of the drug over an extended period, thereby maintaining a consistent plasma drug concentration within the therapeutic window [1]. Among various approaches for achieving sustained release, matrix tablets are one of the most widely employed systems owing to their simplicity, cost-effectiveness, and ease of formulation [2,3].
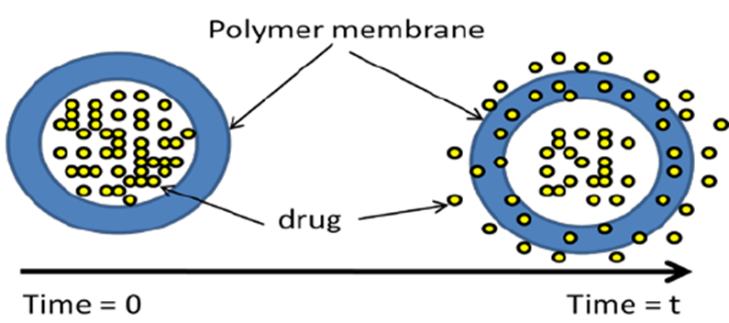
Figure 1: Sustained Release (SR) Matrix Tablet
Matrix tablets are solid oral dosage forms in which the drug is dispersed within a matrix of polymeric materials that regulate the rate of drug release. Depending on the nature of the polymer, matrix systems can be broadly classified into hydrophilic, hydrophobic, and biodegradable types. Hydrophilic matrix systems, in particular, have attracted significant attention due to their ability to control drug release via swelling and diffusion mechanisms. These systems typically employ polymers that absorb water, swell to form a gel layer, and modulate the release of the entrapped drug [4].Matrix tablets are composed of an active pharmaceutical ingredient (API) uniformly dispersed within a polymeric matrix. Upon ingestion, the polymer interacts with gastrointestinal fluids to form a gel layer, which controls the drug release by modulating diffusion and erosion processes [5]. Depending on the type of polymer used, matrix systems can be classified into:
- Hydrophilic matrices, where water-soluble or swelling polymers form a viscous gel upon hydration.
- Hydrophobic matrices, where water-insoluble polymers release the drug primarily by diffusion through pores.
- Biodegradable matrices, which degrade in the body through enzymatic action or hydrolysis, releasing the encapsulated drug in a controlled manner.
Advantages of sustained-release (SR) tablets
- Enhanced Therapeutic Efficacy
- Prolonged Drug Release: Sustained-release tablets maintain therapeutic drug levels in the bloodstream for an extended period, improving the pharmacological response.
- Reduction in Drug Fluctuations: They provide more consistent plasma drug concentrations, minimizing peaks and troughs associated with immediate-release formulations.
- Improved Patient Compliance
- Reduced Dosing Frequency: Patients take these tablets less frequently (once daily or even less), improving adherence to the treatment regimen, especially for chronic conditions.
- Convenience: Simplifies medication schedules, particularly beneficial for elderly patients or those on polypharmacy.
- Minimized Side Effects
- Lower Peak Plasma Concentration: Sustained-release formulations avoid high drug concentration peaks that can cause adverse effects.
- Controlled Drug Exposure: Reducing fluctuations in plasma levels can minimize the risk of dose-dependent toxicity.
- Better Management of Chronic Diseases
- Long-Term Benefits: Ideal for conditions requiring consistent drug levels, such as diabetes, hypertension, and arthritis.
- Reduced Nighttime Dosing: Sustained-release forms can maintain therapeutic levels overnight, benefiting patients with nocturnal symptoms.
- Enhanced Bioavailability
- Bypassing First-Pass Metabolism: For drugs that exhibit extensive first-pass metabolism, SR formulations may provide improved bioavailability by delivering the drug gradually.
- Increased Absorption Time: Allows for better absorption of drugs with a short half-life.
- Economic Benefits
- Cost-Effective in Long Run: Though the initial cost may be higher, sustained-release tablets reduce the frequency of dosing, indirectly saving costs associated with missed doses or frequent doctor visits due to poor compliance.
- Targeted Drug Delivery
- Gastrointestinal Targeting: Some sustained-release systems are designed to release drugs in specific segments of the GI tract for enhanced absorption or action.
- Reduction in Medication Errors
- Simplifies dosing regimens, reducing the risk of patients accidentally taking multiple doses.
- Environmental and Manufacturing Benefits
- Reduction in Packaging Waste: Fewer doses translate to reduced packaging material.
- Optimized Manufacturing: Large-scale production of sustained-release tablets is well-established and cost-effective.
- Improved Stability of Labile Drugs
- Protects Drugs from Degradation: Some sustained-release formulations can protect sensitive drugs from exposure to gastric pH or enzymes [6-10].
Disadvantages of sustained-release (SR) tablets
- Complexity in Formulation
- Advanced Technology Required: The development of sustained-release formulations requires sophisticated technology, advanced materials, and precise control, increasing complexity compared to immediate-release (IR) tablets.
- Difficult Optimization: Achieving consistent release profiles and ensuring uniform drug distribution within the matrix is challenging.
2. Higher Development and Production Costs
- Costly Manufacturing: Specialized processes and equipment increase the cost of production.
- Expensive Excipients: Use of polymers, coatings, or other controlled-release materials adds to the cost.
3. Risk of Dose Dumping
- Sudden Drug Release: If the release mechanism fails (e.g., due to tablet rupture), the entire drug dose can be released at once, leading to toxic plasma levels and adverse effects.
4. Limited Suitability for Certain Drugs
- Short Half-Life Drugs: Drugs with extremely short half-lives require large doses in SR forms, which may not be feasible or safe.
- Poor Solubility Drugs: Drugs with poor solubility can have unpredictable release profiles in SR formulations.
- High Dose Requirement: Drugs requiring large doses may lead to impractically large SR tablets.
5. Individual Variability in Drug Release
- Gastrointestinal Variability: Drug release can be affected by factors such as pH, motility, or transit time in the gastrointestinal (GI) tract, leading to inconsistent therapeutic effects.
- Patient-Specific Factors: Variability in metabolism, co-existing diseases, or food interactions can alter drug release and absorption.
6. Delayed Onset of Action
- Slow Initial Release: Sustained-release formulations may not be suitable for conditions requiring immediate relief, such as acute pain or emergencies.
7. Challenges with Dosage Adjustments
- Limited Flexibility: SR tablets cannot be easily divided or split for dose adjustments, which is problematic for pediatric or elderly patients.
- Difficulty in Titration: Titrating doses in patients with changing therapeutic needs is harder with SR forms.
8. Issues in Disposal and Environmental Impact
- Polymer Waste: Use of synthetic polymers in sustained-release systems can contribute to environmental pollution if not properly managed.
- Drug Residues: Incomplete drug release may leave active drug residues in the tablet, raising concerns about wastage and disposal.
9. Stability Challenges
- Storage Conditions: SR tablets may require strict storage conditions to maintain the integrity of the controlled-release mechanism.
- Moisture Sensitivity: The coating or matrix system can degrade in humid environments, compromising drug release.
10. Regulatory and Quality Control Challenges
- Stringent Regulations: Approval for SR formulations often requires extensive testing to prove safety and efficacy.
- Quality Assurance: Ensuring batch-to-batch consistency is more demanding compared to IR formulations.
11. Incompatibility with Certain Delivery Routes
- Not Suitable for All Routes: Sustained-release tablets are typically limited to oral administration and may not be suitable for drugs that are better delivered via other routes (e.g., parenteral, sublingual).
12. Potential for Reduced Bioavailability
- Incomplete Release: If the drug is not fully released, it can lead to subtherapeutic effects.
- Enzyme Degradation: Drugs susceptible to enzymatic degradation in the GI tract may lose efficacy during extended-release periods [11-13].
Applications of Sustained-Release (SR) Tablets [14-16]Sustained-release tablets have transformed drug delivery by offering prolonged therapeutic effects and enhancing patient compliance. Below are detailed applications of SR tablets, categorized by therapeutic areas.
- Chronic Disease Management
Sustained-release formulations play a critical role in managing chronic conditions such as hypertension, diabetes, and arthritis. For hypertension, drugs like Metoprolol SR and Nifedipine SR maintain consistent blood pressure levels over extended periods, reducing the need for frequent dosing. Similarly, in diabetes management, Metformin SR provides prolonged glycemic control, minimizing the risk of hypoglycemia. In patients with arthritis, NSAIDs like Diclofenac SR and Ibuprofen SR ensure long-lasting pain and inflammation relief, reducing the frequency of dosing and improving quality of life.
2. Pain Management
SR tablets are indispensable in managing both chronic and acute pain. Opioids such as Morphine SR and Oxycodone SR are commonly used in cancer pain management and palliative care, providing prolonged analgesia and reducing breakthrough pain episodes. For post-surgical pain, sustained-release formulations minimize the need for multiple doses, enhancing patient comfort and recovery. These formulations are particularly beneficial in conditions requiring around-the-clock pain relief, such as fibromyalgia and severe arthritis.
3. Neurological Disorders
In epilepsy, sustained-release formulations of antiepileptic drugs like Carbamazepine SR and Valproic Acid SR help maintain therapeutic plasma levels, reducing the frequency and severity of seizures. Similarly, in Parkinson’s disease, SR formulations of Levodopa or Dopamine agonists provide steady drug delivery, improving motor symptom control and reducing the frequency of dosing. These benefits significantly enhance patients’ daily functioning and quality of life.
4. Psychiatric Disorders
SR formulations are widely used in the treatment of psychiatric conditions such as depression and schizophrenia. For instance, antidepressants like Venlafaxine SR and Bupropion SR ensure consistent plasma levels, reducing mood fluctuations and improving adherence. In schizophrenia, SR forms of antipsychotics like Olanzapine provide stable therapeutic effects, minimizing relapse rates and improving long-term management.
5. Cardiovascular Diseases
SR tablets are vital in the management of cardiovascular conditions such as angina pectoris, hypertension, and heart failure. Nitrates like Isosorbide Mononitrate SR provide prolonged anti-anginal effects, reducing the frequency of chest pain episodes. In heart failure management, sustained-release formulations of drugs like Digoxin help maintain therapeutic efficacy while minimizing adverse effects. These advantages make SR formulations a cornerstone in cardiovascular therapy.
6. Infectious Diseases
Antibiotics in sustained-release forms, such as Amoxicillin SR and Ciprofloxacin SR, are used to ensure consistent antibacterial activity over an extended period, improving patient compliance and treatment outcomes. In tuberculosis treatment, sustained-release Rifampicin formulations help maintain effective drug concentrations, which is critical for the success of long-term therapies. These formulations reduce the risk of missed doses, a major concern in treating infectious diseases.
7. Hormonal Therapy
Sustained-release hormonal formulations are used in contraception and hormone replacement therapy (HRT). Birth control pills containing Ethinyl Estradiol and Progesterone in SR forms provide consistent hormone levels, reducing the risk of unintended pregnancies. Similarly, in HRT, sustained-release Estrogens or Androgens help manage symptoms of menopause or hypogonadism more effectively, improving patient satisfaction and adherence.
8. Gastrointestinal Disorders
SR tablets play an important role in managing gastrointestinal conditions such as ulcers, gastroesophageal reflux disease (GERD), and inflammatory bowel diseases (IBD). Proton pump inhibitors like Omeprazole SR ensure prolonged relief from hyperacidity and ulcers. In IBD, drugs like Mesalamine SR release the active ingredient gradually, targeting specific areas of the gastrointestinal tract and providing effective symptom control.
9. Pulmonary Disorders
Sustained-release formulations of bronchodilators, such as Theophylline SR, are used to provide extended symptom relief in asthma and chronic obstructive pulmonary disease (COPD). These formulations improve lung function by ensuring a steady therapeutic effect, reducing the need for frequent dosing and improving patient adherence to treatment regimens.
10. Oncology
In cancer therapy, sustained-release tablets are used to provide consistent plasma levels of chemotherapeutic agents, minimizing the side effects associated with peak drug concentrations. These formulations enhance patient comfort and allow for better management of drug-related toxicities. SR tablets are particularly beneficial in oral chemotherapy regimens, where compliance is critical for therapeutic success.
11. Anticoagulant and Antiplatelet Therapy
Sustained-release formulations of Aspirin and Clopidogrel are used for the long-term prevention of thrombotic events such as stroke and myocardial infarction. By providing controlled drug release, these formulations help maintain therapeutic efficacy while minimizing the risk of bleeding, a common side effect of anticoagulant therapy.
12. Endocrine Disorders
SR tablets are used in the treatment of endocrine conditions such as hypothyroidism and adrenal insufficiency. Levothyroxine SR formulations are being developed to provide consistent thyroid hormone levels, improving patient outcomes. In adrenal insufficiency, sustained-release corticosteroids like Prednisolone help manage symptoms more effectively with less frequent dosing.
13. Lifestyle-Related Applications
SR formulations are gaining popularity in lifestyle-related therapies such as weight management and smoking cessation. Appetite suppressants like Phentermine SR provide prolonged effects, aiding in obesity management. Similarly, Bupropion SR is used to reduce nicotine withdrawal symptoms, improving success rates in smoking cessation programs.
14. Veterinary Medicine
Sustained-release tablets are extensively used in veterinary medicine to reduce dosing frequency in animals. This is particularly useful for long-term treatments of infections, inflammation, or chronic diseases in livestock and pets, where frequent dosing is impractical.
15. Research and Development
SR tablets are often used as a platform for testing novel drug delivery systems, including hydrogels, nanotechnology, and biodegradable matrices. These formulations provide a basis for exploring advanced drug delivery techniques, enhancing the understanding of pharmacokinetics and drug release mechanisms [16,17].
Formulation Methods for Sustained-Release (SR) TabletsSustained-release tablets are formulated using various techniques to achieve controlled and prolonged drug release. Each method has its unique mechanisms, materials, and applications, making them suitable for different types of drugs and therapeutic needs
1. Matrix Systems
Matrix systems are among the most widely used methods for formulating sustained-release tablets. In this approach, the drug is dispersed within a matrix of polymeric material, which controls the release rate [18].
a. Hydrophilic Matrix Systems
Hydrophilic matrices use water-soluble or swellable polymers such as hydroxypropyl methylcellulose (HPMC), guar gum, or hydroxyethyl cellulose. When the tablet comes into contact with gastrointestinal fluids, the polymer swells and forms a gel-like barrier on the surface. This barrier slows the penetration of fluids and the release of the drug. Over time, the gel layer erodes, releasing the drug in a controlled manner. This method is simple to manufacture, cost-effective, and widely used for drugs like Metformin SR, ensuring prolonged therapeutic effects.
b. Hydrophobic Matrix Systems
Hydrophobic matrices consist of water-insoluble materials like ethyl cellulose, waxes, or polyethylene. In these systems, drug release occurs through diffusion from the matrix or erosion of the material. The hydrophobic nature of the matrix makes it particularly suitable for moisture-sensitive drugs or drugs prone to degradation in aqueous environments. Diclofenac SR tablets are an example of this formulation method, offering long-lasting pain relief [18,19].
c. Lipid Matrix Systems
Lipid matrices involve the use of lipophilic substances such as carnauba wax or hydrogenated castor oil. The drug is embedded in the lipid matrix, and release occurs through slow diffusion or erosion of the matrix. Lipid matrices are often employed for poorly water-soluble drugs, as they can enhance the bioavailability and control the release of the active ingredient [20].
2. Reservoir Systems
Reservoir systems are designed with a drug core surrounded by a polymer coating that acts as a barrier to control the release. These systems are known for their precision and ability to provide a steady release rate [21].
a. Coated Tablets
In coated tablet systems, the drug core is coated with polymers such as ethyl cellulose, polymethacrylates (Eudragit), or cellulose acetate. These polymers create a semi-permeable membrane that regulates the diffusion of the drug. Drug release is influenced by the thickness and properties of the coating. Coated tablets like Nifedipine SR are widely used for cardiovascular conditions, providing consistent therapeutic effects with minimal dosing frequency [22].
b. Osmotic Pump Systems
Osmotic pump systems are advanced reservoir systems where the tablet core contains the drug along with an osmotic agent. The core is surrounded by a semipermeable membrane with a laser-drilled hole. When exposed to gastrointestinal fluids, water enters through the membrane, generating osmotic pressure that pushes the drug out through the hole at a controlled rate. This system ensures highly predictable and consistent drug release, as seen in Glipizide extended-release tablets for diabetes management [23].
3. Multi-Unit Systems
Multi-unit systems consist of small, individual units like granules, pellets, or microspheres, which are combined into a single tablet. These systems offer flexibility in drug release and minimize the risk of dose dumping [24].
a. Pellets
Pellets are small spherical units containing the drug, which are coated with release-modifying polymers. These coated pellets are compressed into tablets, and drug release occurs as the polymer coating dissolves or erodes in gastrointestinal fluids. Multi-unit pellet systems (MUPS) provide consistent release profiles and reduce inter-patient variability. Omeprazole delayed-release tablets are an example of this method, effectively protecting the drug from acidic degradation and ensuring prolonged therapeutic effects [25].
b. Microspheres
Microspheres are tiny spherical particles made of polymers such as polylactic-co-glycolic acid (PLGA) or alginate. These particles are incorporated into tablets, and drug release occurs through diffusion or gradual degradation of the microsphere matrix. Microspheres are ideal for poorly soluble drugs, providing extended drug release with high bioavailability [26].
4. Diffusion-Controlled Systems
In diffusion-controlled systems, the release of the drug is regulated by its diffusion through a polymer barrier or matrix. These systems are particularly useful for drugs requiring precise control over release rates [27].
a. Reservoir Diffusion Systems
In reservoir diffusion systems, the drug core is surrounded by a polymer membrane that acts as a rate-controlling barrier. The membrane's permeability and thickness determine the release rate. Drugs like Theophylline SR are formulated using this method, ensuring consistent plasma levels over extended periods [28].
b. Matrix Diffusion Systems
Matrix diffusion systems involve dispersing the drug uniformly within a polymer matrix. The drug release occurs as it diffuses out of the matrix over time. The properties of the matrix material influence the release profile, allowing customization for specific therapeutic needs. Carbamazepine SR tablets use this method to provide steady anti-epileptic effects [29].
5. Other Advanced Techniques
In addition to the methods above, advanced technologies are being developed to further enhance the efficacy of SR tablets. These include:
- Floating Tablets: Designed to remain buoyant in the stomach, ensuring prolonged gastric retention and drug release.
- Bioadhesive Systems: Utilize polymers that adhere to mucosal surfaces, enhancing drug absorption in specific regions of the gastrointestinal tract.
- Nanoparticle-Based Systems: Incorporate nanoparticles to improve the solubility and release profile of poorly soluble drugs [30,31].
Evaluation of Sustained-Release (SR) Tablets
The evaluation of sustained-release (SR) tablets is a comprehensive process designed to ensure that these formulations meet all the necessary quality, safety and efficacy standards. This evaluation involves a variety of tests to assess the physical, chemical, and biological properties of the tablets. Each test helps confirm that the tablets are capable of delivering the drug in a controlled and predictable manner over the desired period.
1. Physical and Mechanical Properties
a. Hardness Test
The hardness test is essential for determining the ability of SR tablets to withstand mechanical stress during handling, packaging, and transportation. This test ensures that the tablet remains intact and does not break easily, which could lead to premature drug release or improper dosing. Hardness is measured using a hardness tester such as the Monsanto or Schleuniger tester, which applies a force to the tablet until it breaks. The result is recorded as the tablet's hardness in terms of kilograms (kg) or Newtons (N). A standard hardness range for SR tablets is typically 4-8 kg, ensuring that the tablets are strong enough to endure physical stress but not too hard to delay dissolution [31,32].
b. Thickness and Diameter
Thickness and diameter measurements are important to confirm uniformity in tablet size. This is especially critical for SR tablets, as variations in size can lead to inconsistent release rates and doses. Uniform tablet size ensures that the drug content and release characteristics are consistent across the batch. Thickness is measured using a vernier caliper or micrometer, and diameter is checked with a digital micrometer. Both parameters should adhere to established specifications with minimal variation to guarantee consistent performance [32].
c. Weight Variation
Weight variation is a test that determines the consistency of tablet weight across a batch. For SR tablets, this test is critical because any deviation from the intended weight can result in differences in the drug content and, consequently, the release profile. During this test, a sample of tablets (usually 20) is weighed individually, and the average weight is calculated. The percentage deviation of individual tablet weights from the mean is then calculated. According to pharmacopeial guidelines, the weight variation should not exceed ±5% for SR tablets to ensure uniformity in drug content.
d. Friability Test
The friability test measures the tablet's tendency to break, chip, or crumble during transportation and handling. This test is vital for SR tablets because excessive friability can lead to broken tablets or loss of controlled-release properties. During this test, tablets are placed in a rotating drum, typically the Roche Friabilator, and subjected to mechanical abrasion for a specified period (usually 100 revolutions). The tablets are then weighed again, and the percentage of weight loss is calculated. A weight loss of less than 1% is acceptable for SR tablets, indicating that the tablets are sufficiently durable without compromising drug release [32,33].
2. In-Vitro Drug Release Testing
In-vitro drug release testing is the cornerstone of evaluating SR tablets. It assesses how effectively and consistently the drug is released from the tablet under controlled conditions that mimic the human gastrointestinal environment.
a. Dissolution Testing
Dissolution testing determines the rate at which the active pharmaceutical ingredient (API) is released from the SR tablet. This is a critical test because it evaluates the formulation's ability to provide the intended therapeutic effect by releasing the drug in a controlled and sustained manner over time. The test is typically conducted using the USP (United States Pharmacopeia) dissolution apparatus. There are two main types of dissolution apparatus used:
- USP Apparatus I (Basket Method): A basket made of mesh is used to hold the tablet, which is immersed in a rotating bath of dissolution medium.
- USP Apparatus II (Paddle Method): The tablet is immersed in a dissolution medium with a rotating paddle. This apparatus is commonly used for SR tablets, as it allows for better control over the release environment.
In SR tablets, the dissolution profile should show a consistent release of the drug over an extended period (e.g., 12-24 hours), rather than a rapid release. For SR formulations, the ideal dissolution profile is a steady, linear release, without any significant burst effect in the initial phase [34].
b. Release Kinetics and Mathematical Models
To analyze the drug release mechanism more thoroughly, the dissolution data obtained from the in-vitro testing is subjected to mathematical modeling. The release profile is compared with various kinetic models to determine the mechanism of drug release, whether it follows zero-order, first-order, Higuchi diffusion, or Peppas models.
- Zero-order kinetics: The drug is released at a constant rate over time, which is ideal for SR tablets.
- First-order kinetics: The release rate is proportional to the remaining amount of drug in the tablet, which is commonly observed for some SR formulations.
- Higuchi model: This model describes drug release from matrices that undergo diffusion, often used to evaluate matrix-based SR tablets.
- Peppas model: A hybrid model that applies when both diffusion and polymer erosion are involved in the release process [34,35].
By applying these models, formulators can understand the release characteristics and optimize the tablet design for the desired release profile.
3. Stability Testing
Stability testing is crucial to ensure that SR tablets maintain their quality, efficacy, and safety throughout their shelf life. Stability studies evaluate how the tablet’s physical properties, chemical composition, and drug release characteristics change over time under various environmental conditions [36].
a. Accelerated Stability Studies
Accelerated stability testing involves storing the tablets under elevated temperature and humidity conditions (e.g., 40°C and 75% relative humidity) for a specified period, usually 6 months. This accelerated process simulates long-term storage and helps to predict the shelf life of the SR tablets. Changes in appearance, hardness, dissolution rate, and drug content are assessed periodically to determine the product's stability [37].
b. Long-Term Stability Studies
Long-term stability studies are conducted under recommended storage conditions (e.g., 25°C and 60% relative humidity) for an extended period (typically 12 months or more). These studies provide comprehensive data on the drug’s stability over time, ensuring that the SR tablet maintains its therapeutic efficacy without degradation. Parameters such as dissolution, drug content, and physical properties (appearance, hardness, and friability) are regularly monitored [38].
c. Moisture Content
Moisture content is a key factor affecting the stability of SR tablets, particularly those with hydrophilic matrix systems. Excess moisture can cause premature drug release or degradation of the API. Moisture content is measured using methods such as the Karl Fischer titration or loss on drying (LOD), which quantifies the water content in the tablets. A low moisture content is typically ideal for SR tablets to prevent degradation and maintain controlled release [39].
4. Drug Content Uniformity
Drug content uniformity is an essential quality control test to ensure that each tablet contains the correct dose of the active ingredient. For SR tablets, the consistency in drug content is particularly important because variations can affect the release profile and therapeutic efficacy. The drug content is determined by extracting the drug from the tablet using suitable solvents and analyzing the concentration using techniques like High-Performance Liquid Chromatography (HPLC) or UV spectrophotometry [40].
5. In-Vivo Evaluation
In-vivo evaluation of SR tablets is conducted to confirm that the in-vitro drug release results correlate with clinical outcomes and therapeutic efficacy. The primary in-vivo evaluations include:
a. Bioavailability Studies
Bioavailability refers to the fraction of the administered dose that reaches the systemic circulation in an active form. Bioavailability studies are essential to demonstrate that the SR tablet delivers the drug effectively to the site of action over the intended duration. These studies measure pharmacokinetic parameters such as:
- Cmax (maximum plasma concentration)
- Tmax (time to reach maximum concentration)
- AUC (area under the concentration-time curve)
For SR tablets, the goal is to achieve a plasma concentration that remains within the therapeutic range for an extended period, without causing high peak concentrations that could lead to toxicity.
b. Bioequivalence Studies
Bioequivalence studies are conducted to compare the pharmacokinetic profiles of the SR formulation with those of a reference product, such as an immediate-release formulation or another marketed SR product. These studies ensure that the new formulation provides the same therapeutic benefits as the reference drug [41,42].
6. Microbiological Evaluation (For Antibiotic SR Tablets)
For SR tablets containing antibiotics or other antimicrobial agents, microbiological testing is essential to confirm the sustained antibacterial activity. This test assesses whether the SR tablet maintains its efficacy against target microorganisms over the duration of its release. Techniques such as minimum inhibitory concentration (MIC) determination and zone of inhibition tests are commonly used to evaluate the antimicrobial potency of the SR formulation [43,44].
CONCLUSION
The development of sustained-release (SR) tablets remains a dynamic and rapidly evolving area of pharmaceutical research, with ongoing innovations and improvements aimed at optimizing drug delivery systems. One of the key trends in this field is the focus on advanced release mechanisms that allow for more precise and controlled drug delivery over extended periods. These include the use of polymers that can respond to environmental changes, such as pH or temperature, and technologies like osmotic pumps that ensure the drug is released at a constant rate. In addition, the move toward personalized medicine, where treatments are tailored to the individual based on factors such as genetic profile and disease condition, is gaining traction. This shift in the healthcare paradigm is leading to the development of SR formulations that can better meet specific patient needs, enhancing therapeutic outcomes. Cutting-edge technologies like 3D printing are also revolutionizing the way SR tablets are formulated, enabling manufacturers to create complex tablet structures with precise drug release profiles, further enhancing the efficacy and safety of these formulations.However, despite the progress in SR tablet formulation, several challenges remain. The complexity of creating SR formulations that deliver a consistent, controlled release of the drug is one of the primary obstacles. Achieving the desired release profile requires meticulous selection of excipients, optimization of polymer systems, and fine-tuning of manufacturing processes. Additionally, gastrointestinal variability such as changes in pH, motility, and enzyme activity can significantly affect the release and absorption of drugs, making it difficult to maintain a predictable therapeutic effect. Drug stability is another critical challenge, as the active ingredient must remain stable over time, even as it is released slowly from the tablet. Interactions between the drug and excipients also need to be carefully considered to prevent degradation or alterations in release rates. Regulatory hurdles are another concern, as the approval process for SR tablets is more stringent compared to conventional tablets, requiring extensive clinical testing to demonstrate safety, efficacy and consistency in release. Moreover, the manufacturing costs for SR tablets are often higher due to the specialized excipients, complex manufacturing processes, and quality control requirements involved. This can make SR tablets less affordable for patients, particularly in low-income regions.Despite these challenges, addressing these issues and embracing emerging technologies offers promising solutions. By leveraging advances in drug delivery systems, formulation technologies, and manufacturing processes, pharmaceutical researchers and formulators can continue to improve the quality and efficacy of SR tablets. With further development, these tablets have the potential to become more accessible, affordable, and effective, ultimately benefiting both patients and healthcare providers. The continuous exploration of innovative techniques, such as the use of natural polymers, biodegradable materials, and personalized drug delivery systems, will likely lead to even more efficient and patient-friendly SR formulations. Through ongoing collaboration, research, and technological advancement, the future of SR tablets looks promising, providing a sustainable and targeted approach to drug therapy that meets the evolving needs of modern medicine
REFERENCE
- Smith J, Doe A. Pharmaceutical formulation strategies for controlled drug delivery systems. J Pharm Sci. 2018;107(5):1234-1243.
- Sharma S, Kumar A, Gupta M. Advances in the development of sustained-release drug delivery systems. J Controlled Release. 2017;255:5-22.
- Patel R, Singh H. A review on novel excipients in sustained-release formulations. Drug Dev Ind Pharm. 2019;45(7):1000-1012.
- Williams V, Brown T. Use of biodegradable polymers in drug delivery. Int J Pharm. 2016;510(1-2):168-177.
- Liu Z, Tang X, Zhang H. The role of microencapsulation in sustained drug release. Drug Delivery Transl Res. 2020;10(4):1125-1138.
- Agarwal A, Das R. Polymeric matrices in controlled release systems. Drug Dev Ind Pharm. 2018;44(2):289-295.
- Singh N, Kumar S. Current trends in osmotic pump technology for drug delivery. J Control Release. 2019;292:85-97.
- Sharma M, Verma V. Microencapsulation of active pharmaceutical ingredients for sustained release formulations. Drug Deliv. 2018;25(1):154-165.
- Kim Y, Lee S. Targeted drug delivery systems: Review and recent advancements. Biomed Pharmacother. 2017;95:373-381.
- Gupta P, Mehta A. Novel methods in sustained release tablets: A review. J Pharm Pharmacol. 2020;72(3):465-472.
- Jones D, Patel M. Polymer-based drug delivery systems for the sustained release of therapeutics. Eur J Pharm Sci. 2017;95:31-42.
- Zheng Y, Chen Q, Liu W. Application of 3D printing technology in controlled release dosage forms. Int J Pharm. 2020;579:119167.
- Roy S, Bandyopadhyay S. Natural excipients for controlled release tablets: A review. J Pharm Investig. 2018;48(5):615-626.
- Kumar M, Gupta S. Design and evaluation of sustained-release tablets: Challenges and perspectives. Acta Pharm. 2019;69(2):169-185.
- Smith R, Jain A. Advances in formulation strategies for sustained-release tablets. Drug Discov Today. 2020;25(12):2210-2219.
- Lee H, Shin S. Manufacturing technologies for sustained-release dosage forms. Pharmaceutics. 2021;13(9):1504.
- Brown J, Thompson P. Regulatory challenges in the development of sustained-release tablets. Drug Dev Ind Pharm. 2019;45(1):99-105.
- Chatterjee S, Raza Z. A review on biocompatible polymers used in controlled release formulations. J Pharm Sci. 2021;110(7):2405-2417.
- Soni A, Agarwal S. Recent advancements in formulation and development of controlled release systems. Drug Dev Ind Pharm. 2020;46(3):211-217.
- Evans M, Klein H. Gastrointestinal considerations in the formulation of sustained-release tablets. Int J Pharm. 2018;544(2):333-344.
- Patel R, Sharma A. The role of nanotechnology in sustained-release drug formulations. Int J Nanomedicine. 2018;13:4311-4322.
- Garg S, Arora S. Tablet-based drug delivery systems for sustained release. Pharm Tech. 2020;44(1):48-58.
- Alam A, Verma K. Natural polymers for sustained release drug formulations: A review. Drug Dev Ind Pharm. 2019;45(6):800-809.
- Zeng W, Liu M. Advances in the formulation of controlled release oral dosage forms. Int J Pharm. 2017;518(1-2):218-227.
- Banerjee S, Roy B. Controlled drug delivery: Recent developments and future challenges. Eur J Pharm Sci. 2019;138:105-112.
- Wu Y, Yang X. Polymeric materials for sustained release formulations: A review. Mater Sci Eng C. 2020;109:110453.
- Zheng W, Wang X. Applications of biopolymers in sustained drug release systems. Biomaterials. 2018;182:69-86.
- Singh S, Soni M. Technological advances in drug delivery for controlled release systems. Int J Pharm. 2017;527(1-2):115-127.
- Kumar R, Mehta V. Pharmaceutical formulations for sustained drug release: Challenges and perspectives. Curr Drug Deliv. 2020;17(7):725-736.
- Rao S, Sharma M. Sustained release dosage forms for targeting chronic diseases. J Chronic Dis. 2019;62(10):736-744.
- Gupta M, Kumar R. Controlled release drug delivery systems: Design and application. Drug Discov Today. 2017;22(7):1093-1104.
- Bansal A, Agarwal V. Development of polymeric matrix systems for sustained release drugs. Pharmaceutics. 2021;13(10):1745.
- Reddy P, Kumar S. Review of formulation techniques and challenges in sustained-release tablets. Drug Dev Ind Pharm. 2020;46(9):1286-1295.
- Sen A, Bhagat R. Impact of manufacturing variables on sustained-release drug formulation. Pharm Tech. 2021;45(2):35-42.
- Zhang Y, Tan Q. Investigating the influence of excipients on sustained drug release profiles. J Pharm Sci. 2020;109(2):537-549.
- Lee H, Shin S. Targeted and controlled drug delivery systems: The future of personalized medicine. J Pharm Investig. 2021;51(5):615-625.
- Patel S, Kumar P. Drug delivery systems in modern pharmaceuticals: Recent trends and innovations. Drug Dev Ind Pharm. 2021;47(4):493-505.
- Gupta A, Yadav K. Polymers in controlled drug delivery systems: A review. Int J Pharm. 2018;537(1-2):305-315.
- Song X, Wang M. Polymers for sustained release drug formulations: Recent advancements. J Mater Chem B. 2019;7(13):2091-2102.
- Yang Y, Li Y. Microencapsulation for controlled drug release: Mechanisms and applications. J Control Release. 2020;322:315-328.
- Sharma P, Mehta S. Recent trends in 3D printing of pharmaceutical formulations. J Pharm Sci. 2021;110(9):2955-2965.
- Tiwari R, Kumar G. Natural biopolymers for the development of sustained-release drug formulations. Carbohydr Polym. 2020;235:115950.
- Kim S, Park J. Sustained-release oral drug delivery systems: Technology and challenges. J Pharm Innov. 2018;43(2):113-124.
- Kumar A, Srivastava S. Formulation and characterization of sustained-release tablets. Indian J Pharm Sci. 2020;82(3):425-436


 Mangesh Dagale*
Mangesh Dagale*

 10.5281/zenodo.14678430
10.5281/zenodo.14678430