Abstract
Hydrogels are hydrophilic, Three-dimensional polymeric networks are known as hydrogels. Different synthetic and natural polymers cross-link chemically or physically to generate hydrogels. One of the main components of the human body that is used in biomedicine is water. Polymers are having significance role in the pharmaceutical field. Hydrogels are considered biomaterials because of their significant qualities, which include non-toxicity, biocompatibility, and biodegradability. These attributes allow hydrogel to be used in the pharmaceutical and medical fields. This paper's main objective is to review the literature on the technologies related to the production, classification, and application of hydrogels. The pharmacological and therapeutic restrictions on hydrogels for use in biomedical applications have been greatly addressed in recent years. This article's main goal is to review the hydrogels' classification according to their physical and chemical properties. It also goes over the technology used to create hydrogels and the various uses for them in the contemporary world.
Keywords
hydrogel, cross linkages, application, co -polymer, drug delivery
Introduction
Hydrogels are networks made of polymers that can absorb and retain large amounts of water. These polymers have hydrophilic (water-attracting) groups that get hydrated when in contact with water, resulting in the formation of the hydrogel structure. (Akhtar et al.,2016) Hydrogels were initially introduced by Wichterle and Lím in 1960 (Nautiyal et al.,2019) Hydrogels can be either chemically stable or can break down and dissolve over time. (Wichterle & Lim et al.,1960) Hydrogels are also known as 'reversible' or 'physical' gels if entanglements and/or secondary forces like ionic, hydrogen bonding, or hydrophobic forces are primarily responsible for generating the connection, hydrogels are also referred to as "reversible" or "physical" gels. Physical gels can be dissolved by adjusting environmental factors like pH, solution ionic strength, and temperature. Physical gels are frequently reversible. Crosslinking polymers in a dry state or in solution can achieve the coupling of covalent bonds connecting different macromolecular chains in "permanent" or "chemical" gels (Peppas et al.,2000) Almost every water-soluble polymer, with a variety of bulk physical characteristics and chemical compositions, can be used to create hydrogels. Hydrogels can also be created in a different of physical forms, including coatings, films, microparticles, and slabs (C. Rosiak & Yoshii et al.,1999) Hydrogels can be produced using numerous "classical" chemical methods. These comprise both multi-step processes involving the synthesis of polymer molecules with reactive groups and their subsequent cross-linking, as well as one-step processes like polymerization and parallel cross-linking of multifunctional monomers by reacting polymers with appropriate cross-linking agents. (Van et al.,2003) Mucilage and gums found in nature are made up of several ingredients. Polysaccharides, resins, and their derivatives, including hydrogels, can impart distinct physical and chemical properties in a variety of dosage forms and applications. Because they can hold large amounts of water, hydrogels can replicate the properties of extracellular matrix. Three-dimensional hydrophilic polymeric networks, or hydrogels, are able to imbibe vast amounts of liquids, including biological fluids that closely resemble biological tissues. Due to this characteristic, these systems have generated a lot of interest in the design of novel devices for modulated systems by adjusting their physicochemical features, which can be tweaked. (ahmad et al.,2018) Hydrogels' ability to form "in-situ" using less invasive techniques is one of their other key features. A number of techniques can be used to create hydrogels using the crosslinking of polymer chains idea. (Sharma et al.,2017) Polymer crosslinking can be achieved through chemical modification, grafting, crosslinker application, or high energy radiation (UV or gamma rays). Hollow core-shell particles and hydrogels with a variety of compositions, morphologies, and sizes are produced using a conservative and expert radical polymerization process. The use of macroinitiators and functional initiators strengthens the function of functional groups within or on the hydrogels' surface, facilitating multivalent bioconjugation. (Hennink and van et al.,2012) The different techniques used by different researchers or scientists to create hydrogels fall into two groups: physical and chemical crosslinking techniques. While physical interactions between the hydrogel's polymer chains existed, chemical approaches involve the creation of new covalent bonds between them. (Bhardwaj et al.,2000) (Sharma et al.,2018) Every crosslinking technique has advantages and disadvantages of its own. It is noteworthy to mention that hydrogels produced using mild physical crosslinking conditions typically perform worse when compared to counterparts created using covalent crosslinking techniques. These hydrogels are stabilized by relatively weak connections between networks of polymers, such as ionic, hydrophobic, or hydrogen bonding. (ali and ahmed et al.,) Natural materials, particularly natural gums, can be used to create hydrogels using both chemical and physical processes. Consequently, there is a need to substitute natural additives for synthetic ones in light of the current tendencies towards the application of plant-based natural goods. Numerous natural materials derived from plants are being researched for their potential applications in different fields such as therapeutics and pharmaceutics. Because hydrogels, also known as smart or hungry networks, have the potential to be used in biomedical technology transfers and exhibit a notable physicochemical change in response to minute changes in the environment, they are currently the focus of scientific investigation. Since these modifications are reversible, the unique characteristic is the ability to revert to the starting state following a response as soon as the trigger is removed. (Noreen et al.,2017) (singh et al.,2010).

Fig 1. Structure of hydrogel (Yadav & Madan et al.,2020)
Mechanism Of Hydrogel Formation
Chemically crosslinked hydrogels have been researched because of their high availability, biocompatibility, and changeable functional groups. Polymers are frequently used to make chemical and physical hydrogels (Mathew et al., 2018). Hydrogels can be customized for a specific purpose by selecting the appropriate kind of polymer or monomer and hydrogel forming reactions. Mechanical strength. Chemical and physical crosslinking are the two processes that create hydrogels (wang and han et al.,2017)
Chemical Crosslinking
One kind of hydrogel that can be changed from a liquid to a solid via covalent bonding is called a chemically cross-linkable hydrogel. Hydrogel systems installed in situ also employ this technique. This technique forms hydrogels by combining a number of events, including optical polymerisation, enzyme reactions, and click reactions. The previously mentioned techniques for creating these hydrogels will be covered in this section. (Kurnia et al.,2012) Hydrogels that are chemically crosslinked have been studied because of their high mechanical strength.(Akhtar et al.,2016a)
Optical Polymerization
Optical polymerization is one method of chemical crosslinking in hydrogen synthesis that has the advantages of minimal energy consumption and no solvent requirement for the reaction. This method makes use of light-sensitive substances included in hydrophilic polymers. When the polymer solution is exposed to UV or visible light, free radicals and the optical decomposition initiator are created, which starts the polymerization process. Polymers that crosslink in this fashion usually have groups of acrylates and methacrylate, which are polymerized by light. With this technique, the rate of gelling may be regulated, and the hydrogen produced can be utilized to release medicinal substances like proteins and medications. (Kurnia et al.,2012a) One of the best techniques for converting a monomer into a polymer using an optical initiator is this one. (chamkouri and chamkouri et al.,2021) Optical polymerization, which strengthens the hydrogel and allows the cells to fade during gel formation, is used in clinical applications. The hydrogel also acquires a porous lattice structure as a result of this process.(Monteiro et al.,2018) Optical polymerisation can be effortlessly carried out within or outside the body at physiological pH and temperature. A non-toxic optical primer with the right wavelength of light from a light source should be utilised for hydrogels used in the medical industry. One such process is optical crosslinking, which happens when UV light causes vinyl groups to break. (mironi et al.,2012)
Physical Crosslinking
By altering intramolecular forces such hydrogen bonding, hydrophobic interaction, and electrostatic ionic force, hydrogels created by physical bonding can be produced. This method allows hydrogel to be prepared using safe and easy procedures, preventing the potential rise in the toxicity of the crosslinker in the chemical method. Ionic, pH-dependent, and temperature-dependent cross-linking techniques are examples of physical cross-linking techniques. (guaresti et al.,2018)
Ionic Crosslinking
One of the physical crosslinking techniques uses an ion crosslinking agent to generate a gel, and in this manner, Without a covalent bond developing between the polymer chains, the crosslinking reaction is released. (Mironi and others, 2018a) (Kretlow and others, 2007) Hydrogels have a great degree of durability because of this process. With its polymer solution, the natural polysaccharide alginate can create gels that are resistant to divalent cations such as Ca2+. These ions lead to the formation of guluronic acid groups in the alginate chain and ionic bonds within the polymer chain. Hydrogen alginate is a common ECM type. More recently, researchers have focused on improving mechanical properties, gelling time, and biological linkages. Through the manipulation of variables including molecular weight, alginate and calcium concentrations, and alginate composition in the hydrogel, they can be ready for application in injectable biomaterials and in vitro culture. (wang and han et al.,2017a)
Types Of Hydrogels:
Based on the natural and synthetic polymers utilised in their creation, hydrogels have been divided into several categories.
1.Natural Hydrogels:
Hydrogels are made from natural polymers such as collagen, fibrin, gelatine, etc. Natural polymers have several beneficial qualities, including biologically recognised moieties that facilitate cellular activity and inherent biocompatibility and biodegradability. These polymers, however, fall short in providing adequate mechanical characteristics, and they could harbour infections or trigger an inflammatory or immunological reaction (Davis & Anseth et al.,2002) (Lin & Metters et al.,2006)
2.Synthetic Hydrogels:
Synthetic materials utilised for hydrogel synthesis include polymers like polyvinyl alcohol (PVA), polyethylene oxide (PEO), and polyacrylic acid (PAA). Synthetic polymers possess unique properties such as precisely engineered structures that can be altered to produce specific degradability and functionality. Hydrogels' structure can be changed by adjusting their chemical makeup and manufacturing processes. However, unlike natural polymers, manufactured hydrogels lack intrinsic bioactive characteristics. (Peppas et al.,2000a) (Lin and Metters et al.,2006a)
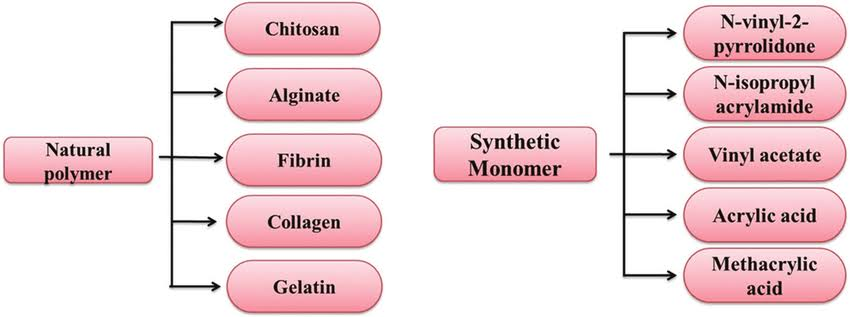
Fig. 2: Distinct types of hydrogels based on polymer type(Chauhan et al.,2012)
ADVANTAGES (Reddy et al.,2017)
1. Hydrogel has greater strength and elasticity.
2. Hydrogel is easily modifiable and has good transparency qualities.
3. They are quite flexible, much like genuine tissue, because of their high-water content.
4. They can be injected and are both biocompatible and biodegradable.
5. Hydrogels can detect changes in pH, temperature, or metabolite concentration and release their load in response to such changes.
6. Timely release of nutrients or medications.
DISADVANTAGES (Mohite & Adhav et al.,2017)
1. Expensive.
2. Non-adherent; may require supplementary dressing for security; may also induce sensation due to maggot movement.
3. Difficult to clean.
4. In hypoxia, dehydration, and red eye reactions in contact lens less deposition.
Properties Of Hydrogel:
The application of hydrogels, or hydrophilic gels, in the fields of pharmaceutical and biomedical engineering is receiving a lot of interest.
- Swelling Properties: Rapid & reversible changes taken place in hydrogel can be triggered by very minor environmental changes. The presence of an enzyme or other ionic species, temperature, pH, electric signal, and other external factors can all alter and affect the hydrogel's physical texture. (Das et al.,2013)
- Mechanical properties: The purpose of the material can affect and modify its mechanical qualities. By heating the material, the degree of crosslinking can be increased or decreased to produce a gel with greater stiffness. A variety of factors and reasons are linked to changes in mechanical characteristics, therefore varying analyses are required depending on the material. (Chirani et al.,2016)
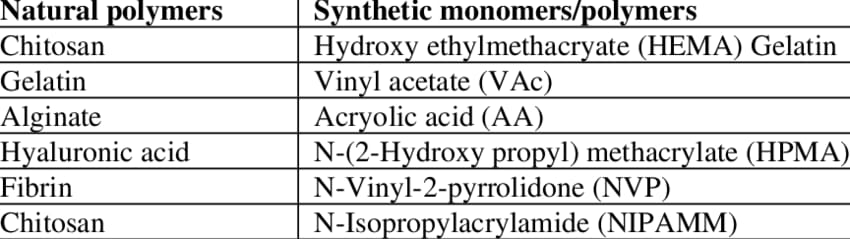
- Polymers used in hydrogel preparation: Both synthetic and natural polymers are employed to make hydrogels.
Table.1 Natural polymers and synthetic monomers used in hydrogel fabrication (Putri et al.,2020)
Classification Of Hydrogels:
Numerous hydrogel classifications and points of view are reported in the literature. The primary building blocks for hydrogels are polyelectrolytes and/ or biopolymers. According to the source, hydrogels can be classified into two categories grounded on their composition those made of natural polymers and those made of synthetic polymers.( Gehrke and lee et al., 1990) Hydrogels have three possible states cationic, anionic, and neutral, depending on the ionic charges on the bonded groups. The orders may also be grounded on the kinds ofcross-linking agents. Physical, chemical, or biological hydrogels are all possible. When temperature, ionic concentration, pH, or other environmental factors change, or when two components are mixed together, physical gels may pass a phase transition from a liquid to a gel. Hydrogels can also be classified into amorphous, semi-crystalline, crystalline, and hydrocolloid aggregates according to their structural makeup. (Silva.et al., 2009)
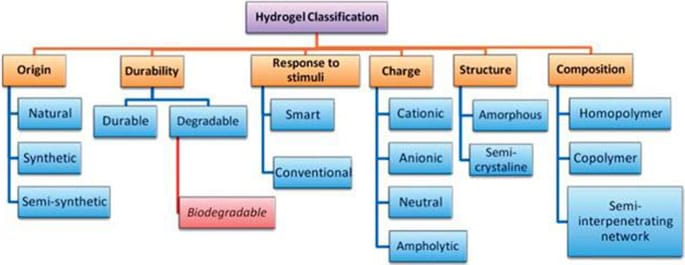
- Classification based on source Hydrogel can be divided into two group:
Natural origin and synthetic origin.
- Classification based on the polymeric composition
1.Homopolymeric hydrogels: Hydrogels made of homopolymeric polymers are referred to as polymeric networks that originate from a single species of monomer. The cross-linked skeletal structure of these hydrogels is dependent on the type of monomers and polymerization process used. (Yadav and madan et al.,2015a)
2.Copolymeric hydrogels: These hydrogels consist of distinct monomers, each containing a single hydrophilic component. (sowjanya et al.,2013)
3.Multipolymer Interpenetrating polymeric hydrogels (IPN):
It consists of two separate components made of either synthetic or natural polymers. One polymer in this kind of classification is a noncross-linked polymer network.
- According to the biodegradability (shetye et al.,2015)
1. Biodegradable hydrogels:
Hydrogels are biodegradable Several polymers formed by nature are biodegradable, such as Chitosan, fibrin and agar. Poly (aldehyde guluronate), Polyanhydrides and poly (N-isopropyl acrylamide) are examples of manufactured biodegradable polymers.
2.Non-biodegradable hydrogels:
Non-biodegradable hydrogels are commonly prepared using a variety of vinylated monomers or macromers, including 2-hydroxyl propyl methacrylate, methoxy poly (ethylene glycol), 2-hydroxyl ethyl methacrylate, and acrylamide.
- Classification based on configuration (meshram et al.,2017)
The classification of hydrogels depends on their physical structure and chemical composition can be classified as follows:
1. Amorphous (non-crystalline).
2. Semi crystalline: A complex mixture of amorphous and crystalline phases.
3. Crystalline.
- Classification based on type of cross-linking (Malpure et al.,2018)
Depending on whether the cross-link junctions are chemical or physical, hydrogels can be classified into two groups.
1.Networks that are chemically cross-linked feature permanent connections.
2. Transient junctions in physical networks can result via hydrophobic or hydrogen bonding interactions, or they might be caused by polymer chain entanglements.
- Classification based on physical appearance
The method of polymerization used in the preparation process determines whether a hydrogel appears as a matrix, film, or microsphere.
- Classification according to network electrical charge (meshram et al.,2017a)
Depending on whether the cross-linked chains in hydrogels have an electrical charge or not, they can be divided into four types.
1. Non-ionic (neutral).
2. Ionic (including anionic or cationic).
3. Amphoteric electrolyte (ampholytic) containing both acidic and basic groups.
4. Zwitter ionic (polybetaines) containing both anionic and cationic groups.
APPLICATION:
- Wound Healing
Hydrogels' cross-linked nature gives them the capacity to contain both drugs and water. Wound exudates can be held and retained by them because of their capacity of holding water. Polyacrylamide or polyvinyl pyrrolidine in the form of a gel that is 70–95% water (Khapare et al.,2016)
- Drug delivery in GI tract:
Drugs are delivered to specific GIT sites using hydrogels. Drugs loaded with colon-specific hydrogels exhibit tissue specificity, a change in pH, or an enzymatic action that degrades the medication when microflora is present. (Remington et al.,2006)
- Rectal Delivery:
For rectal drug delivery, hydrogels with bio adhesive qualities are employed. (sri et al.,2012)
- Tissue Engineering:
Antigen-presenting cells' cytoplasm can receive macromolecules through the use of micronized hydrogels. For tissue engineering, natural hydrogel materials similar as agarose, methylcellulose, and other naturally generated substances are utilised. (Calo and Khutoryanskiy et al.,2015)
- Ocular drug delivery:
The most common use of hydrogels is in ocular drug delivery systems. Hydrogels parade sustained or controlled release, which can lower the frequence of dosing or boost medicine effectiveness by localising the medicine at its point of action, lowering the dosage demanded, or delivering harmonious medicine delivery. (Dubey and prabhu et al., 2014)

Figure 4: Applications of hydrogels. (Amir et al., 2018a)
Other Applications Of Hydrogels:
- Perfume delivery: The number of patents outlining technologies for delivering unpredictable species began to increase in the 1990s. Specifically, Procter & Gamble appears to have issued the most important patents in the sector, which include turning perfumes into cyclodextrin complexes. The overall goal was to produce gadgets that could release perfumes into the terrain gradationally over time, and to replace traditional swab- grounded tablets( sodium dodecyl benzene sulphonate) with further advanced, useful, and, let's admit it, upmarket household cleaning products. Watering beads for plants: Hydrogels can be applied simply by using rough powders of potassium polyacrylate or polyacrylamide matrix, which are marketed under a wide variety of names, including Super Crystals, Water-Gel, and Plant Gel crystals and utilized as a long-term water storage facility for home and occasionally commercial horticulture.
- Diapers: Over the past 20 years, the development of hydrogel-containing diapers the majority of which are packed with various sodium polyacrylate formulations has significantly reduced the incidence of dermatological diseases linked to extended contact with moist tissues.
- Cosmetics: The cosmetics sector is at the forefront of hydrogel technology; in fact, a pH-sensitive polymer called P(MAA-co-EGMA) has been developed for the release of pharmaceuticals used in cosmetics, such as niacinamide, arbutin, and adenosine, which are well-known compounds for skin whitening and wrinkle treatment.
- Environmental applications: Water contamination is one of the main problems that underprivileged areas, in particular, face. Hydrogels can be utilized to remediate water sources in two different ways because of their affinity for water. First, the matrix can be utilized to contain and cleanse microorganisms. Capturing microorganisms within a variety of carrier materials has led to the development of many fascinating investigations along this specific path. Among them, chlorella and spirulina are most commonly used. These microbes are formerly employed to purge poisons that contaminate water inventories. Hydrogels that were natural and synthetic were both employed. Alginate-derived hydrogels, or carrageenan and agar, seem to be the most effective hydrogels reported in the literature. Changing the hydrogels to allow them to absorb and retain the pollutant is an intriguing second strategy for addressing the pollution issue.
- Plastic Surgery: The extracellular matrix-like qualities of the hydrogel made them suitable materials to utilize in contact with the human body. This is the primary rationale behind initiatives to use hydrogels as novel materials for plastic restoration Hydrogels also show pledge as bulking agents for the treatment of urinary incontinence. In clinical procedures, smart injectable gels can be employed to constrict the urethral channel and lessen incontinence in cases. (Neethu et al.,2018)
CONCLUSION:
Hydrogel based delivery devices can be used for oral, ocular, epidermal, subcutaneous application due to their high-water contents and soft consistency hydrogels resemble natural living tissue more than any other class of synthetic biomaterials. Recently, many hydrogel-based networks have been designed and personalized to meet the needs of different applications. In semi solid formulation ointment, lotion, gels, and topical solution are frequently used and hydrogels have various physical and chemical properties, swelling, mechanical properties, cross linking, biocompatible properties, porosity and permeation. Hydrogels have played a significance role in biomedical field i.e. drug delivery, tissue engineering, wound dressing, environmental, bacterial culture, hydrogel to fix for bone replacement therapy.Because hydrogels have a soft consistency and a high water content, they can be employed for oral, ophthalmic, epidermal, and subcutaneous applications.
REFERENCE
-
-
-
- Akhtar MF, Hanif M, Ranjha NM. Methods of synthesis of hydrogels… A review. Saudi Pharmaceutical Journal. 2016 Sep 1;24(5):554-9.
- Nautiyal U, Devi A, Charanjeet AS. Development and evaluation of interpenetrating polymer network hydrogel for controlled release of cefadroxil. Int. J. Health Biol. Sci. 2019;2(3):01-15.
- Wichterle O, Lim D. Hydrophilic gels for biological use. Nature. 1960 Jan 9;185(4706):117-8.
- Peppas NA, Bures P, Leobandung WS, Ichikawa H. Hydrogels in pharmaceutical formulations. European journal of pharmaceutics and biopharmaceutics. 2000 Jul 3;50(1):27-46.
- Rosiak JM, Yoshii F. Hydrogels and their medical applications. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 1999 May 2;151(1-4):5664.
- Van der Linden HJ, Herber S, Olthuis W, Bergveld P. Stimulus-sensitive hydrogels and their applications in chemical (micro) analysis. Analyst. 2003;128(4):325-31.
- Ahmad M, Manzoor K, Ahmad S, Ikram S. Preparation, kinetics, thermodynamics, and mechanism evaluation of thiosemicarbazide modified green carboxymethyl cellulose as an efficient Cu (II) adsorbent. Journal of chemical & engineering data. 2018 May 22;63(6):1905-16.
- Sharma G, Naushad M, Kumar A, Rana S, Sharma S, Bhatnagar A, Stadler FJ, Ghfar AA, Khan MR. Efficient removal of coomassie brilliant blue R-250 dye using starch/poly (alginic acid-cl-acrylamide) nanohydrogel. Process Safety and Environmental Protection. 2017 Jul 1; 109:301-10.
- Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Advanced drug delivery reviews. 2012 Dec 1; 64:223-36.
- Bhardwaj TR, Kanwar M, Lal R, Gupta A. Natural gums and modified natural gums as sustained-release carriers. Drug development and industrial pharmacy. 2000 Jan 1;26(10):1025-38.
- Sharma G, Thakur B, Naushad M, Kumar A, Stadler FJ, Alfadul SM, Mola GT. Applications of nanocomposite hydrogels for biomedical engineering and environmental protection. Environmental chemistry letters. 2018 Mar; 16:113-46.
- Ali A, Ahmed S. Recent advances in edible polymer-based hydrogels as a sustainable alternative to conventional polymers. Journal of agricultural and food chemistry. 2018 Jun 7;66(27):6940-67.
- Noreen A, Akram J, Rasul I, Mansha A, Yaqoob N, Iqbal R, Tabasum S, Zuber M, Zia KM. Pectins functionalized biomaterials; a new viable approach for biomedical applications: A review. International journal of biological macromolecules. 2017 Aug 1; 101:254-72.
- Singh A, Sharma PK, Garg VK, Garg G. Hydrogels: a review. Int. J. Pharm. Sci. Rev. Res. 2010 Sep;4(2):97-105.
- Yadav S, Madan J. Hydrogels: A review. International Journal of Pharmacy & Life Sciences. 2020 Jun 1;11(6).
- Mathew AP, Uthaman S, Cho KH, Cho CS, Park IK. Injectable hydrogels for delivering biotherapeutic molecules. International journal of biological macromolecules. 2018 Apr 15; 110:17-29.
- Wang K, Han Z. Injectable hydrogels for ophthalmic applications. Journal of Controlled Release. 2017 Dec 28; 268:212-24.
- Kurnia JC, Birgersson E, Mujumdar AS. Analysis of a model for pH-sensitive hydrogels. Polymer. 2012 Jan 24;53(2):613-22.
- Chamkouri H, Chamkouri M. A review of hydrogels, their properties and applications in medicine. Am. J. Biomed. Sci. Res. 2021 Feb;11(6):485-93.
- Monteiro N, Thrivikraman G, Athirasala A, Tahayeri A, França CM, Ferracane JL, Bertassoni LE. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dental materials. 2018 Mar 1;34(3):389-99.
- Mironi-Harpaz I, Wang DY, Venkatraman S, Seliktar D. Photopolymerization of cell-encapsulating hydrogels: crosslinking efficiency versus cytotoxicity. Acta biomaterialia. 2012 May 1;8(5):1838-48.
- Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Advanced drug delivery reviews. 2007 May 30;59(4-5):263-73.
- Guaresti O, García–Astrain C, Aguirresarobe RH, Eceiza A, Gabilondo N. Synthesis of stimuli–responsive chitosan–based hydrogels by Diels–Alder cross–linkingclick reaction as potential carriers for drug administration. Carbohydrate polymers. 2018 Mar 1; 183:278-86.
- Davis KA, Anseth KS. Controlled release from crosslinked degradable networks. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2002;19(4-5).
- Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Advanced drug delivery reviews. 2006 Nov 30;58(12-13):1379-408.
- Sandeep C, Harikumar SL, Kanupriy A. Hydrogels: a smart drug delivery system. Int. J. Res. Pharm. Chem. 2012;2(3):603-14.
- Reddy KV, Nagabhushanam MV, Naik ER. Swellable hydrogels and cross-linking Agents-Their role in drug delivery system. Research J. Pharm. and Tech. 2017 Mar 28;10(3):937-43.
- Mohite PB, Adhav SS. A hydrogels: Methods of preparation and applications. Int. J. Adv. Pharm. 2017;6(3):79-85.
- Das N. Preparation methods and properties of hydrogel: A review. Int. J. Pharm. Pharm. Sci. 2013 Jan;5(3):112-7.
- Chirani N, Yahia LH, Gritsch L, Motta FL, Chirani S, Fare S. History and applications of hydrogels. Journal of biomedical sciences. 2015;4(02):1-23.
- Putri GN, Manikam NR, Andayani DE. Asia-Pacific Journal of Oncology Nursing.
- Gehrke SH, Lee PI. Hydrogels for drug delivery systems. Drugs and the pharmaceutical sciences. 1990; 41:333-92.
- Silva AK, Richard C, Bessodes M, Scherman D, Merten OW. Growth factor delivery approaches in hydrogels. Biomacromolecules. 2009 Jan 12;10(1):9-18.
- Amir M, Vohra M, Sharma A, Wadhwa S. Journal of Pharmaceutical Technology Research and Management.
- Sowjanya P, Komali BV, Babu CA. A review article on hydrogels. Int J Res Pharm Nano Sci. 2013;2(5):548-53.
- Shetye SP, Godbole A, Bhilegaokar S, Gajare P. Hydrogels: Introduction, preparation, characterization and applications. Hum. J. 2015 Oct; 1:47-71.
- Meshram PS, Kale SD, Labale PS, Mate KS. Hydrogel Polymer: A Unique Material for Bio-Separation, Bio-Sensing and Drug Delivery. International Advanced Research Journal in Science, Engineering and Technology. 2017;4(3).
- Malpure PS, Patil SS, More YM, Nikam PP. A review on-hydrogel. Am J PharmTech Res. 2018;8(3):42-60.
- Khapare SS, Bhandare MG, Talele SG, Jadhav A. An Emphasis on Hydrogels for Pharmaceutical Applications. American Journal of PharmTech Research. 2016;6(3).
- Remington JP. Remington: the science and practice of pharmacy. Lippincott Williams & Wilkins; 2006.
- Sri B, Ashok V, Arkendu C. As a review on hydrogels as drug delivery in the pharmaceutical field. Int J Pharm Chem Sci. 2012;1(2):642-1.
- Calo E, Khutoryanskiy VV. Biomedical applications of hydrogels: A review of patents and commercial products. European polymer journal. 2015 Apr 1; 65:252-67.
- Dubey A, Prabhu P. Formulation and evaluation of stimuli-sensitive hydrogels of timolol maleate and brimonidine tartrate for the treatment of glaucoma. International journal of pharmaceutical investigation. 2014 Jul;4(3):112.
- Neethu TM, Dubey PK, Kaswala AR. Prospects and applications of hydrogel technology in agriculture. International Journal of Current Microbiology and Applied Sciences. 2018;7(5):3155-62.


 Mayuri Jagtap*
Mayuri Jagtap*





 10.5281/zenodo.14737267
10.5281/zenodo.14737267